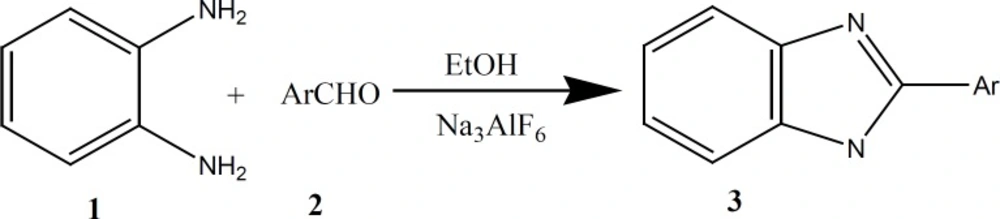
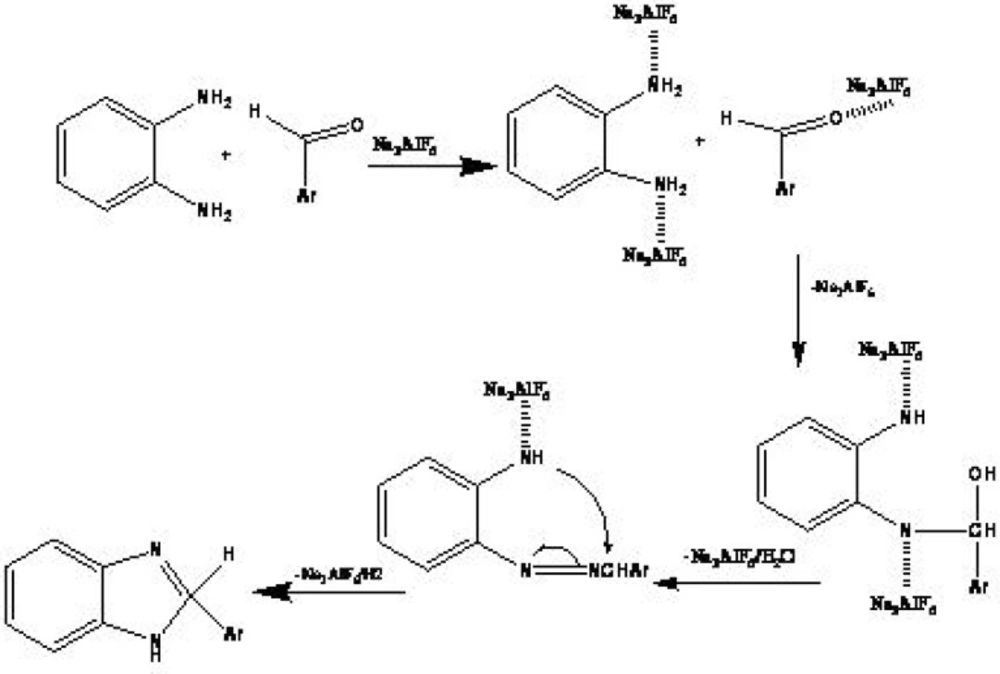
Introduction
Benzimidazole and imidazole derivatives are very useful bioactive intermediates for the preparation of pharmalogical and biological active molecules (1-6). This ring system is present in numerous antiparasitic, fungicidal, antithelemintic and anti-inflammatory agents (4-7). There are two general methods for the synthesis of 2-substituted benzimidazoles (8-19). The most general method is a condensation reaction of o-phenylenediamine with carboxylic acids or their derivatives such as nitriles, imidates or ortho esters (10), under strongly acid conditions or in combination with very high temperatures and/or microwave irradiations. The other one includes an oxidative cylocondensation reaction of Schiff bases in turns by condensation of o-phenylenediamine with aldehyde (6, 8, 9). A variety of oxidants and catalysts have been used for preparation of benzimidazoles. Although these methods worked nicely in many cases, however, some of these suffer from one or more limitations such as low yields, use of volatile or toxic organic solvents, requirement of excess amounts of catalysts or reagents, special apparatus and harsh reaction conditions. Consequently, development of convenient, high yield and environmentally benign procedure for synthesis of benzimidazoles is still a challenging research.
Sodium hexafluroaluminate (Na3AlF6), known as cryolite, is a non-toxic and commercially available compound that can be used as a catalyst in the laboratory without special precautions.
Due to the important biological activity of benzimidazoles and in line with our research works in synthesis of this ring system (20-24) we wish to report a simple procedure for preparation of 2-arylbenzimidazoles through a condensation reaction of o-phenylenediamine and aromatic aldehydes in the presence of Na3AlF6 as a catalyst. We also report the antibacterial activity of these compounds.
Experimental
Melting points were determined on an electrothermal digital melting point apparatus and are uncorrected. The IR spectra were recorded on Unicom Galaxy series FT-IR 5000 spectrometer. NMR spectra were recorded on a Bruker Avance (300 MHz) spectrometer. Chemical shifts (ppm) were referenced to the internal standards tetramethylsilane (TMS). Microanalyses were performed by the Elemental Analyzer (Elemental, Vario EL III). The Microanalyses results were agreed favorably with the calculated values. Reactions were monitored by thin layer chromatography using silica gel F254 aluminum sheets. Almost all synthesized compounds are known and identified using IR and NMR spectroscopy and also by comparison with their authentic samples.
General procedure for preparation of 2-arylbenzimidazoles(3a-n)
To a solution of o-phenylenediamine (1 mmol) and corresponding aromatic aldehyde (1 mmol) in ethanol (20-25 mL) was added Na3AlF6 (2 mol%). The reaction mixture was stirred at 50 C for desired time. The progress of reaction was monitored by TLC. After completion of reaction for appropriate time (Table 3), water (3-40 mL) was added to give the crystals, which then filtered and washed with cold water and air dried.
Antibacterial study
We used the agar disk diffusion test or Kirby-Bauer disk-diffusion method. The microbial strains are identified strains and were obtained from the Pasteur Institute of Iran. The bacterial strains studied are Staphylococcus aureus (RTCC, 1885), and Escherichia Coli (ATCC, 35922). Each chemically synthesized material (5 mg) was dissolved in 250 µL of DMSO (20 µg/µL) and 100 µL of the solution of the test compounds was introduced onto the disks (0.7 cm diameter). The disks were then placed on top of the medium previously inoculated with bacteria. 100 µL of solvent (DMSO) was added to another disk and implanted as a negative control on each plate along with the standard drugs. The plates were incubated overnight at 37 °C. The inhibition zones were measured and compared with the standard drugs. For the zone size interpretations were used recommendations of the National Committee of Clinical Laboratory Standards (NCCLs). The results are given in Table 4. The inhibition zone numbers are the average of three times independent experiments.
2-Phenyl-1H-benzimidazole (3a)
IR (KBr): ν= 3348 (NH), 3047 (CHaromatic), 1462 (C=C) cm-1. 1HNMR (300 MHz, DMSO): δ ppm = 7.20-7.65 (m 7H, CHaromatic), 8.16-8.20 (m, 2H, CHaromatic), 12.93 (bs, 1H, NH). Anal. Calcd. For C13H10N2: C, 80.39; H, 5.19; N, 14.42%. Found: C, 80.58; H, 5.40; N, 14.51%.
2-(4-Methylphenyl)-1H-benzimidazole (3b)
IR (KBr): ν = 3323 (NH), 3059 (CHaromatic) 1448 (C=C), 1622 (C=N) cm-1. 1HNMR (300 MHz, DMSO): δ (ppm) = 2.38 (s, 3H, CH3), 7.17-8.08 (m, 8H, CHaromatic), 12.83 (bs, 1H, NH). Anal. Calcd. For C14H12N2: C, 80.74; H, 5.81; N, 13.45%. Found: C, 80.71; H, 6.02; N, 13.39%.
2-(2-Nitrophenyl)-1H-benzimidazole (3c)
IR (KBr): = 3364 (NH), 3024 (CH aromatic), 1446 (C=C), 1518 (N=O) cm-1. 1H NMR (300 MHz, acetone-d6): δ (ppm) = 7.25-8.05 (m, 8H, aromatic), 13.06 (bs, 1H, NH). Anal cald for C13H9N3O2: C, 65.27; H, 3.79; N, 17.56%. Found: C, 65.01; H, 3.96; N, 17.74%.
2-(3-Nitrophenyl)-1H-benzimidazole (3d)
IR (KBr): = 3358 (NH), 3086 (CH aromatic), 1431 (C=C), 1516 (N=O) cm-1. 1H NMR (300 MHz, acetone-d6): δ (ppm) = 7.28-9.05 (m, 8H, aromatic), 12.21 (bs, 1H, NH). Anal cald for C13H9N3O2: C, 65.27; H, 3.79; N, 17.56%. Found: C, 65.51; H, 3.59; N, 17.63%.
2-(4-Nitrophenylhenyl)-1H-benzimidazole (3e)
IR (KBr): ν = 3367 (NH), 3061 (CHaromatic) 1516 (C=C), 1597 (C=N) cm-1. 1HNMR (300 MHz, DMSO): δ ppm = 7.254-7.75 (m, 4H, CHaromatic), 8.39 (bs, 4H, CHaromatic), 13.30 (bs, 1H, NH). Anal. Calcd. For C13H9N3O2: C, 65.27; H, 3.79; N, 17.56%. Found: C, 65.39; H, 3.58; N, 17.47%.
2-(4-Bromophenyl)-1H-benzimidazole (3g)
IR (KBr): ν = 3350 (NH), 3051 (CHaromatic) 1429 (C=C) cm-1. 1HNMR (300 MHz, DMSO): δ 7.20-8.12 (m, 8H, CHaromatic), 13.0 (bs, 1H, NH). Anal. Calcd. For C13H9BrN2: C, 57.17; H, 3.32; N, 10.26%. Found: C, 57.23; H, 3.52; N, 10.34%.
2-(2-Chlorophenyl)-1H-benzimidazole (3h)
IR (KBr): ν = 3377 (NH), 3061 (CHaromatic) 1440 (C=C), 1599 (C=N) cm-1. 1HNMR (300 MHz, DMSO): δ ppm = 7.21 (q, J= 2.6 Hz, 2H, CHaromatic), 7.501-7.54 (m, 3H, CHaromatic), 7.66 (q, J= 3.8 2H, Hz, CHaromatic) 7.90 (t, 1H, J= 9.4 Hz, CHaromatic), 12.73 (bs, 1H, NH). Anal. Calcd. For C13H9N2Cl: C, 68.28; H, 3.97; N, 12.25%. Found: C, 68.40; H, 4.05; N, 12.38%.
2-(3-Chlorophenyl)-1H-benzimidazole (3i)
IR (KBr): = 3354 (NH), 3045 (CH aromatic), 1442 (C=C), 744 (C-Cl). 1HNMR (300 MHz, acetone-d6): δ ppm = 7.24-8.27 (m, 8H, aromatic), 12.00 (bs, 1H, NH). Anal cald for C13H9N2Cl: C, 68.28; H, 3.97; N, 12.25%. Found: C, 68.07; H, 4.10; N, 12.03%.
2-(4-Chlorophenyl)-1H-benzimidazole (3j)
IR (KBr): = 3342 (NH), 3055 (CH aromatic), 1448 (C=C), 746 (C-Cl) cm-1. 1HNMR (300 MHz, acetone-d6): δ ppm = 7.18-8.21 (m, 8H, aromatic), 13.00 (bs, 1H, NH). Anal cald for C13H9N2Cl: C, 68.28; H, 3.97; N, 12.25%. Found: C, 68.03; H, 4.21; N, 12.41%.
2-(2-Hydroxy-5-bromophenyl)-1H-benzimidazole (3k)
IR (KBr): ν = 3364 (NH), 3065 (CHaromatic) 1436 (C=C) cm-1. 1HNMR (300 MHz, acetone-d6): δ ppm = 5.31 (s, 1H, NH), 6.75-7.54 (m, 7H, aromatic), 10.28 (bs, 1H, NH). Anal cald for C13H9N2BrO: C, 54.00; H, 3.14; N, 9.69%. Found: C, 54.23; H, 3.36; N, 9.82%.
2-(3-Metoxyphenyl)-1H-benzimidazole (3l)
IR (KBr): ν = 3051 (CHaliphatic), 2924 (CHaromatic), 1602 (C=N), 1464 (C=C) cm-1. 1HNMR (300 MHz, DMSO): δ 3.07 (s, 3H, OCH3), 7.46-8.22 (m, 8H, CHaromatic) 13.30 (bs, 1H, NH). Anal. Calcd. For C14H12N2O: C, 74.98; H, 5.39; N, 12.49%. Found: C, 75.10; H, 5.31; N, 12.59%.
2-(4-Metoxyphenyl)-1H-benzimidazole (3m)
IR (KBr): ν = 3481 (NH), 3132 (CHaromatic) 1437-1502 (C=C), 1622 (C=N) cm-1. 1HNMR (300 MHz, DMSO): δ ppm = 3.82 (s, 3H, OCH3), 7.11-7.17 (m, 2H, CHaromatic), 7.21 (q, J= 3.2 Hz, 2H, CHaromatic), 7.54 (t, J= 3.0 Hz, 2H, CHaromatic), 8.16 (t, J= 8.9 Hz, 2H, CHaromatic) 12.75 (bs, 1H, NH). Anal. Calcd. For C14H12N2O: C, 74.98; H, 5.39; N, 12.49%. Found: C, 74.79; H, 5.46; N, 12.29%.
2-(3,5-Dimethoxyphenylhenyl)-1H-benzimidazole (3n)
IR (KBr): ν = 2849 (CHaliphatic) 3055 (CHaromatic) 1504 (C=C), 1606 (C=N) cm-1. 1HNMR (300 MHz, DMSO): δ 3.84 (s, 3H OCH3), 3.894 (s, 3H OCH3), 7.12-7.77 (m, 7H, CHaromatic), 12.76 (bs, 1H, NH). Anal. Calcd. For C15H14N2O2: C, 70.85; H, 5.55; N, 11.02%. Found: C, 70.96; H, 5.41; N, 11.12%.
Results and Discussion
Reactions were carried out by taking a 1:1 mol ratio mixture of o-phenylenediamine with aromatic aldehydes 2 in the presence of Na3AlF6 in ethanol to give 2-arylbenzimidazoles (Figure 1). However, aliphatic aldehydes such as formaldehyde or acetaldehyde were also tested under the same conditions, but the corresponding products were isolated in trace amounts.
For optimization of the amount of catalyst required for this reaction, p-nitrobenzaldehyde was used as a model compound and different amounts of catalyst were tested under the same conditions. It was found that 2 mol% of catalyst was enough for a fairly high yield (Table 1). On the other hand, an amount of catalyst over than 2 mol% did not improve the yield of product.
| Entry | Mol% Catalyst | Time (h) | Yield (%) |
|---|---|---|---|
| 1 | 2 | 2 | 80 |
| 2 | 4 | 2.1 | 80 |
| 3 | 5 | 2.5 | 80 |
| 4 | 7 | 3 | 80 |
Reaction of o-phenylenediamine with p-nitrobenzaldehyd in ethanol using different amounts of catalyst at 50 C.
To examine the effect of solvent for this model reaction, we have also performed the reaction in various organic solvents at room temperature with 2 mol% of Na3AlF6. As Table 2 shows, ethanol is more suitable solvent for this procedure. Consequently the reaction was carried out in ethanol with 2 mol% of Na3AlF6 for the preparation of benzimidazoles (3a-n). The results are summarized in Table 3.
| Entry | Solvent | Time (h) | Yield (%) |
|---|---|---|---|
| 1 | Ethanol | 2 | 80 |
| 2 | Methanol | 2.3 | 60 |
| 3 | DMF | 3 | 60 |
| 4 | DMSO | 3.5 | 64 |
| 5 | Acetonitrile | 3.5 | 44 |
Reaction of o-phenylenediamine with p-nitrobenzaldehyd using using different solvents, prompted by 2 mol%Na3AlF6 at 50 C
| Product (3) | Ar | Time (h) | Yield (%) | M.P. (C) |
|---|---|---|---|---|
| a | C6H5 | 11 | 80 | 282-284 (290-292)a |
| b | 4-CH3C6H4 | 9 | 81 | 264-266 (268-270) a |
| c | 2-NO2C6H4 | 17 | 68 | 269-271 (264-266)b |
| d | 3-NO2C6H4 | 13 | 72 | 205-207 (203-204)c |
| e | 4-NO2C6H4 | 2 | 80 | 310-312 (312-314) a |
| f | 3-BrC6H4 | 7.5 | 75 | 248-250 |
| g | 4-BrC6H4 | 4 | 92 | 284-296 (283-284)b |
| h | 2-ClC6H4 | 15 | 60 | 231-232 (232-234) a |
| i | 3-ClC6H4 | 8 | 68 | 232-234 (234-236)b |
| j | 4-ClC6H4 | 16 | 83 | 293-294 (291-293) a |
| k | 2-HO,5-BrC6H3 | 1 | 96 | 207-208 (256-257)b |
| l | 3-OCH3C6H4 | 13 | 95 | 204-206 |
| m | 4-OCH3C6H4 | 14.5 | 80 | 222-224 (225-226) a |
| n | 3,5-(OCH3)2 C6H3 | 16 | 62 | 232-233 |
Reaction of o-phenylenediamine with aromatic aldehydes, prompted by 2 mol% Na3AlF6 in C2H5OH at 50 C
A possible mechanism, supported by literatures (25), has proposed for this reaction. The reaction may prompt by an interaction between the carbonyl group and Na3AlF6 as shown in Scheme 1. The 1H and 13C NMR spectra as well as the elemental analyses data of all synthesized compounds are consistent with the expected structures. The 1H NMR spectra of benzimidazoles (3a-n) consists of a multiplet and a broad singlet at downfield shift resulting from the aromatic protons and the NH group, respectively.
The investigation of antibacterial screening data revealed that the compounds 3e, 3j, 3k and 3n show antibacterial activities against E. coli, as gram negative bacteria (Table 4). Also compounds 3a, 3b, 3e, 3i, 3j, 3k, 3l and 3n, showed good inhibition against S. aureus as compared to penicillin zone of inhibition. The anti-S. aureus activity of compounds 3j, 3k and 3n with inhibition zone of 36, 43 and 32 mm, respectively, are better than that of other compounds.
| Compound (3) | Staphylococcus aureus (mm) | Escherichia coli (mm) |
|---|---|---|
| a | 21 | – |
| b | 23 | – |
| c | – | – |
| d | – | – |
| e | 18 | 13 |
| f | - | – |
| g | - | – |
| h | – | – |
| i | 20 | – |
| j | 36 | 14 |
| k | 43 | 27 |
| l | 16 | – |
| m | – | – |
| n | 32 | 10 |
| DMSO | – | – |
| Standard drugs | Penicillin 33 mm | Gentamicin 18 mm |
1 Zone inhibition of benzimidazoles (3a-n).
In conclusion, we have developed a simple and high efficient procedure for the synthesis of 2-arylbenzimidazoles with advantages of operational simplicity, good to high yields and use of non-toxic and commercial available catalyst.