Introduction
Currently, there is an increased attention for the use of new molluscicides which are highly effective, rapidly biodegradable, less expensive, readily available and probably easily applicable with simple techniques than synthetic molluscicides. One of the new trends in the biological control of vectors of diseases is testing the toxicity of plant extracts, as alternatives to chemical molluscicides, which proved to be environmentally safe and have less residual activity. There are many restrictions of using toxic compounds (pesticides and molluscicides) with fresh water. Therefore, the safety of plant extracts to human being is an advantage for studying their effect against the snail vectors of schistosomiasis.
The botanical molluscicides are of economic importance, especially in developing countries (1). Also, there is a continuous need to search for new plant species with ideal molluscicidal properities (2-3). Different plants have been reported as molluscicides (4-7). In Egypt, screening of local plants for molluscicidal activity has received increasing attention (8-13).
The treatment of B. alexandrina snails with sublethal concentration of C. lanceolatus was effective in altering the amino acid profile of this snail species which could be contributed to the impairment of snails egg laying capacity, snail-schistosome miracidiae finding mechanisms and immune response of the molluscan hosts but has no effect on the mammalian skin penetration rate by schistosome cercariae (14). The genus Callistemon was reported to contain diverse chemical profile; steroids and triterpenes (15 - 18), flavonoids (15, 19 and 20), tannins and phenolic compounds (15, 19 and 21), tetradecahydroxanthenediones (22), in addition to the essential oils (23, 24). The diversity of chemical constitution of different Callistemon species reflects diversity in its biological activities; antibacterial and antifungal activities (25, 26), molluscicidal activity (14), Bio-repellents for land leeches, insecticidal and anthelmintic (27- 29), in addition to antioxidant and hepatoprotective activity (21), antithrombin (30), antidiabetic (31), anti-inflammatory (32), anti- alzheimers disease (20).
Reviewing the current literatures, C.viminalis was not previously investigated for mulloscicidal activity. So, the aim of the present study is to evaluate the efficacy of the methanolic extracts of C. viminalis (Sol. Ex Gaertner) G.Don Ex Loudon fruits, bark and leaves as molluscicides against Biomphalria alexandrina snails. Some histological parameters in snails tissues were determined.
Experimental
Materials and methods
Collection of snails
Fresh water snails were collected by using ordinary snail traps with long hand (33) from the irrigation and drainage canals in Mansoura, Dakahlia Governorate. The snails were individually isolated and placed in plastic bags with a suitable amount of water from the same source and immediately transported to the laboratory (34). The snails were identified according to Ibrahim et al. (35).
Maintenance of snails
Laboratory bred B. alexandrina snails were used in the work. The snails were washed several times and maintained in dechlorinated tap water to acclimatize to the laboratory conditions for four weeks before experimentation (36). The average size of the used adult snails was 10-14 mm in diameter. The snails were kept separately in plastic aquaria, 8-10 liters capacity, in dechlorinated tap water allowing 10 snails/liter to avoid crowding. The aquaria contained no mud, sand, gravel or any other substratum. Dechlorinated water was obtained by storing tap water in large containers for at least 48 hours up to one week. Water in the aquaria was changed as frequent as required to keep the snails in a good condition for experiments. Dead snails were removed every day, and no artificial aeration was used. The snails were fed on fresh boiled lettuce leaves, and they were kept under natural illumination. The temperature was the ordinary room temperature with its natural diurnal fluctuations (24-26 ± 2). Maintenance of snails was established in Parasitology Department, Faculty of Medicine, Mansoura University.
Plant materials
C. viminalis (Sol. Ex Gaertner) G.Don Ex Loudon fruits, bark and leaves. The plant identity was confirmed by Dr. Ibrahim Mashaly; Professor of systematic botany, Faculty of Science, Mansoura University, Egypt.
Preparation of plant extracts
The shade-dried fruits, bark and leaves (1 Kg, each) were powdered and extracted by percolation in two liters percolator with distilled methanol. The combined methanol extracts of each part were concentrated to a syrupy consistency under reduced pressure and then allowed to dry in a desiccator over anhydrous CaCl2. The dried extracts were kept for testing as molluscicides.
Bioassay
Ten adults B. alexandrina of the same size were introduced in plastic acquaria (25×13×7 cm) with the selected concentration of the tested extract. Three grams of each extract were transferred into 100 mL volumetric flasks, and then diluted with dechlorinated tap water to complete the volume. These solutions were used to prepare the required concentrations. Different concentrations of the tested extracts (10, 20 and 40 ppm) were used at three replicates and three containers were left as control. The results were recorded every 24 hours up to 5 days for the number of dead snails. Death of the snails was determined by lack of movement, lack of response to gentle prodding with a blunt object, discoloration of the shell, absence of bleeding when they are crushed, and finally by the standard method of immersing the snails in a small amount of 5% sodium hydroxide in a Petri-dish. If bubbles and blood come out of the shell, it is recorded as alive, and Vice-Versa (34, 37 - 39). After 24 hours, LC50 values for each extract were calculated according to Litchfield and Wilcoxon (40). The percentage of dead snails was calculated according to Abbot's formula, 1952:
Histological preparations
Treated and control snails were removed from their shells, washed thoroughly with distilled water and fixed in 10% formalin. The snail tissues were processed for paraffin sectioning after embedded in paraplast at 50 oC. The 7 µm sections were stained with iron haematoxylin and eosin, and examined for tissue changes with light microscopy.
Results and Discussion
Effect of the tested extracts on the snails
In Tables 1, 2, 3 and 4, the results of the effect of different C. viminalis extracts are listed. LC25 and LC50 values for each extract were listed in Table 5. Death of snails began after 24 hours from application at low concentrations. In the control experiment, one snail was died after 24 hours, while with the tested extracts at least 8 snails were died. This indicates the molluscicidal activity of the titled plant. Complete death of snails was observed after the third day with the extracts of bark and leaves at concentrations 20 and 40 ppm, while a concentration 10 ppm afforded 66.7% and 63.3% mortality for the extracts of bark and leaves respectively. On the other hand, complete death of snails was observed after the first day with the fruits extract even at a concentration 10 ppm. LC50 values for C. viminalis fruits bark and leaves were 6.2, 32 and 40 ppm respectively. Thus, C. viminalis fruits extract has the highest potential to kill the snails at low concentration and in short periods. According to the World Health Organization of plant molluscicide, screening must kill snails after 24 hours in a concentration 100 ppm or less at constant water temperature (39).
| Time (day) | Number of snails | Control | |
|---|---|---|---|
| Death | % | ||
| 1 | 30 | 1 | 3.3 |
| 2 | 30 | 2 | 6.7 |
| 3 | 30 | 2 | 6.7 |
| 4 | 30 | 3 | 10 |
| 5 | 30 | 3 | 10 |
Death of snails in the control experiment
| Time (day) | Number of snails | 5 ppm | 10 ppm | 20 ppm | 40 ppm | ||||
|---|---|---|---|---|---|---|---|---|---|
| Death | % | Death | % | Death | % | Death | % | ||
| 1 | 30 | 10 | 33.3 | 30 | 100 | 30 | 100 | 30 | 100 |
| 2 | 30 | 17 | 56.7 | 30 | 100 | 30 | 100 | 30 | 100 |
| 3 | 30 | 21 | 70 | 30 | 100 | 30 | 100 | 30 | 100 |
| 4 | 30 | 26 | 86.7 | 30 | 100 | 30 | 100 | 30 | 100 |
| 5 | 30 | 29 | 96.7 | 30 | 100 | 30 | 100 | 30 | 100 |
Effect of C. viminalis fruits extract on B. alexandrina snails (number 30).
| Time (day) | Number of snails | 10 ppm | 20 ppm | 40 ppm | |||
|---|---|---|---|---|---|---|---|
| Death | % | Death | % | Death | % | ||
| 1 | 30 | 9 | 30 | 12 | 40 | 17 | 56.66 |
| 2 | 30 | 16 | 53.3 | 23 | 76.7 | 25 | 83.3 |
| 3 | 30 | 20 | 66.7 | 30 | 100 | 30 | 100 |
| 4 | 30 | 25 | 83.3 | 30 | 100 | 30 | 100 |
| 5 | 30 | 28 | 93.3 | 30 | 100 | 30 | 100 |
Effect of Callistemon viminalis bark extract on Biomphalaria alexandrina snails (number 30).
| Time (day) | Number of snails | 10 ppm | 20 ppm | 40 ppm | |||
|---|---|---|---|---|---|---|---|
| Death | % | Death | % | Death | % | ||
| 1 | 30 | 8 | 26.7 | 13 | 43.3 | 15 | 50 |
| 2 | 30 | 15 | 50 | 21 | 70 | 24 | 80 |
| 3 | 30 | 19 | 63.3 | 30 | 100 | 30 | 100 |
| 4 | 30 | 23 | 76.7 | 30 | 100 | 30 | 100 |
| 5 | 30 | 27 | 90 | 30 | 100 | 30 | 100 |
Effect of C. viminalis leaves extract on B. alexandrina snails (number 30).
| C. viminalis extract | LC50 |
|---|---|
| Fruits | 6.2 |
| Bark | 32 |
| Leaves | 40 |
LC50 values for C. viminalis extracts against B. alexandrina snails
In the control test, 3 snails out of 30 were dead during the period of the experiment (10%). These results are in agreement with the results of Youssif et al. (41) who reported that the daily mortality of B. alexandrina was 2.2%.
Histopathological changes
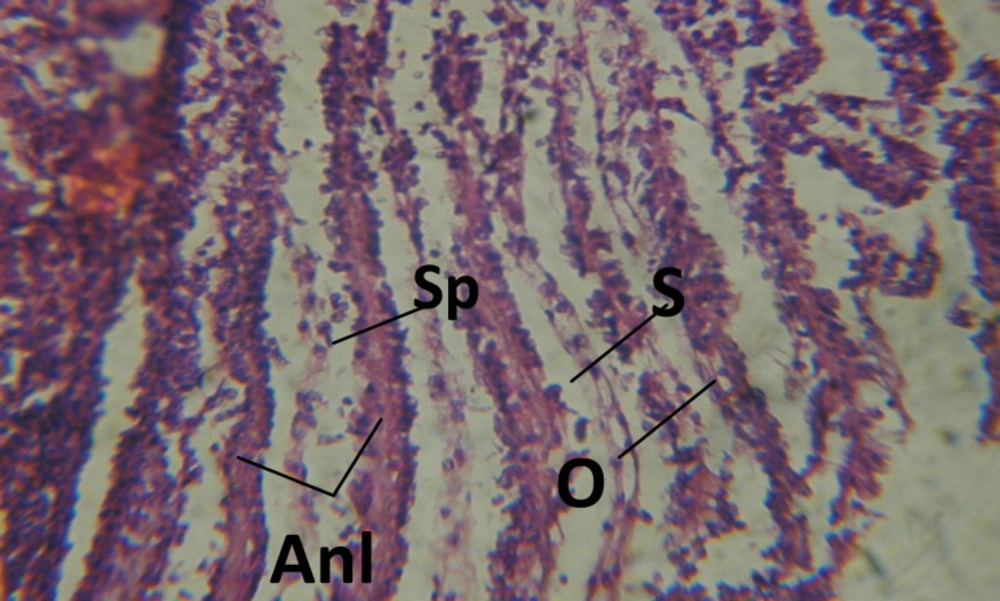
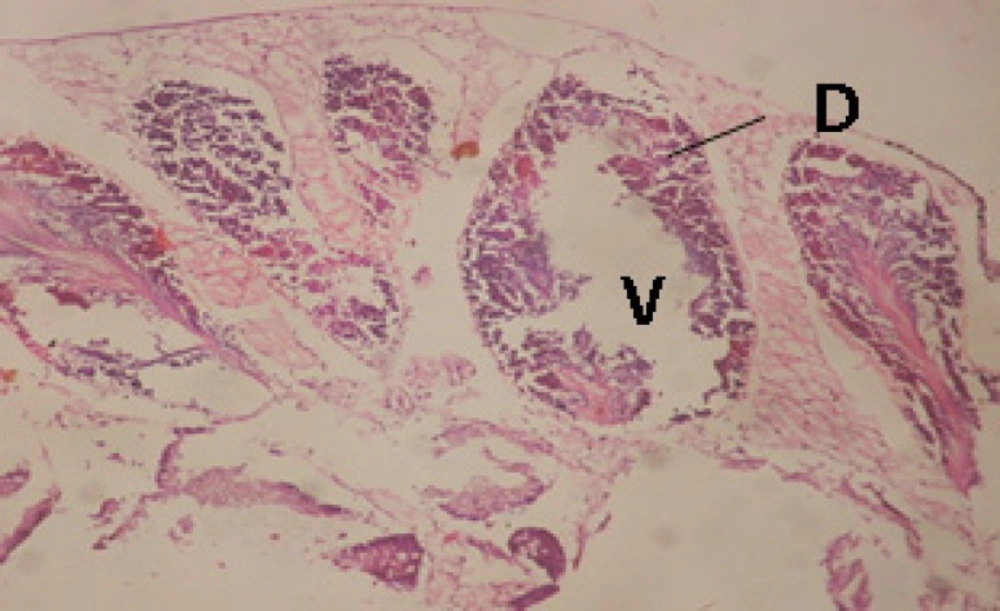
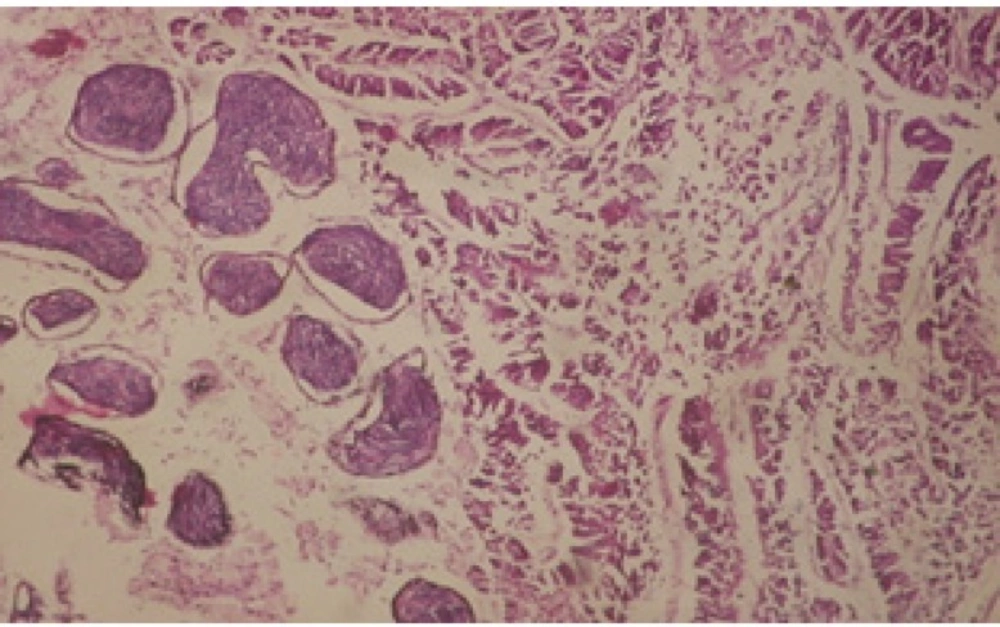
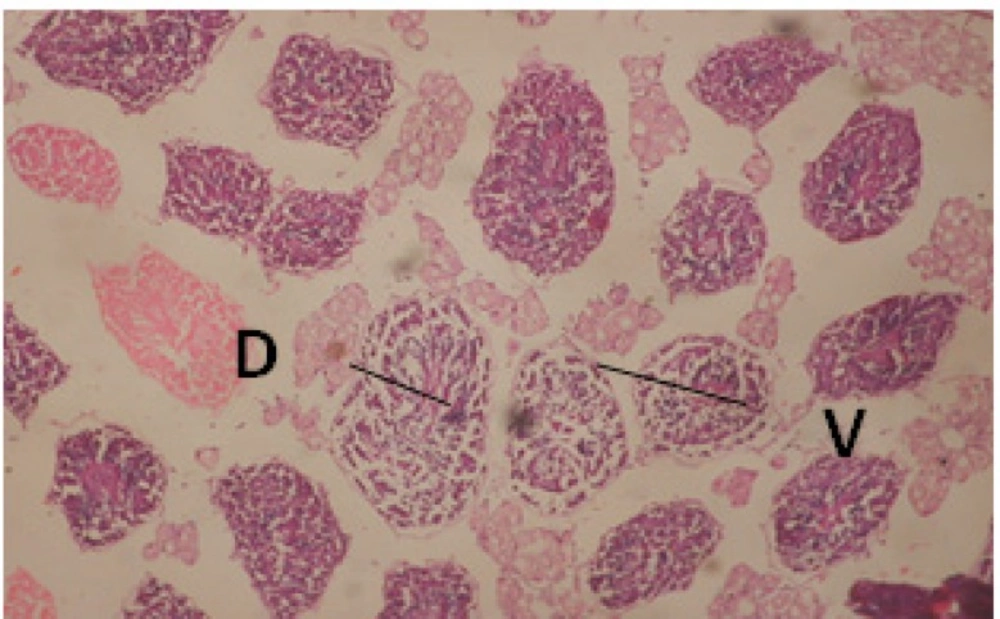
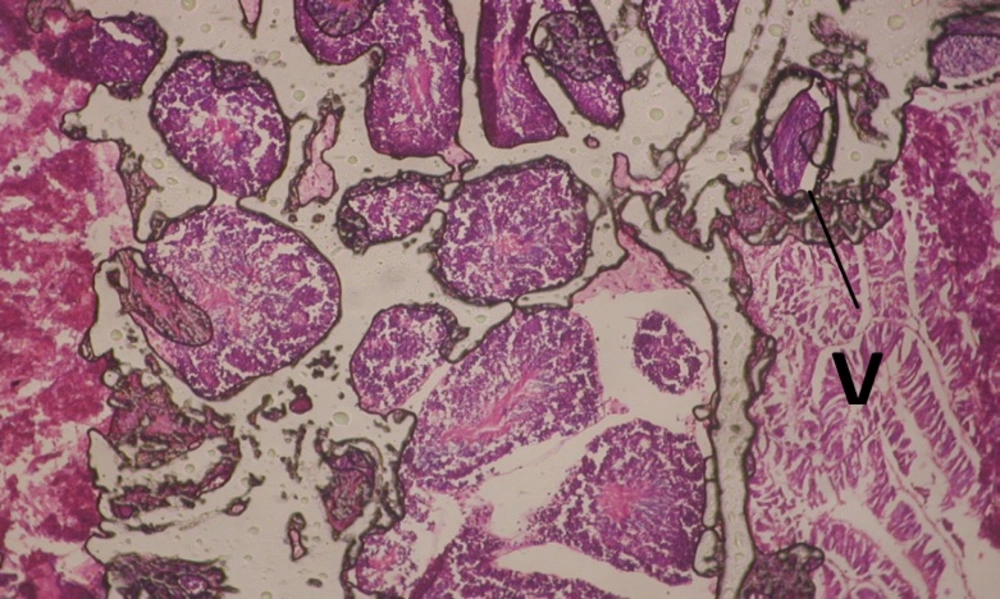
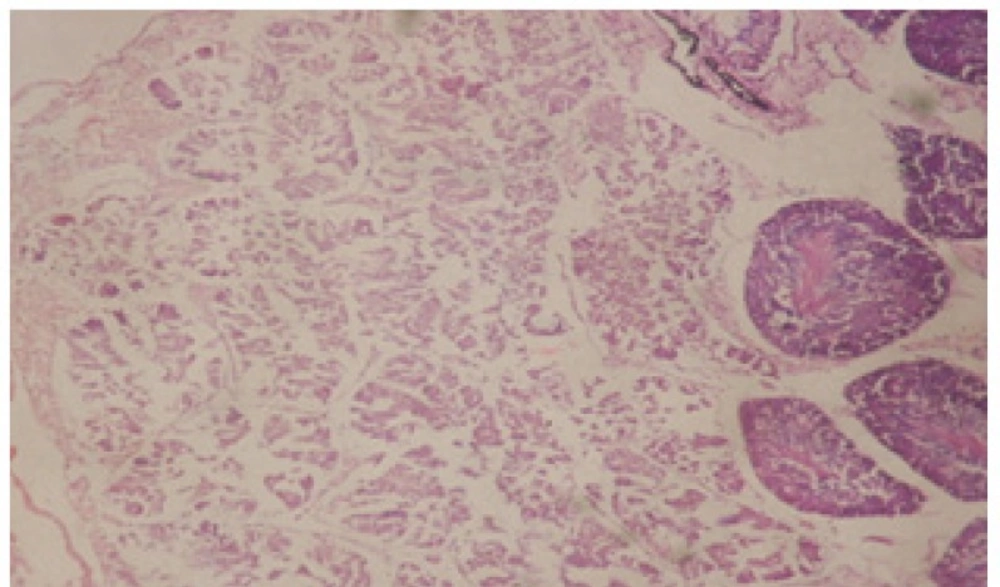
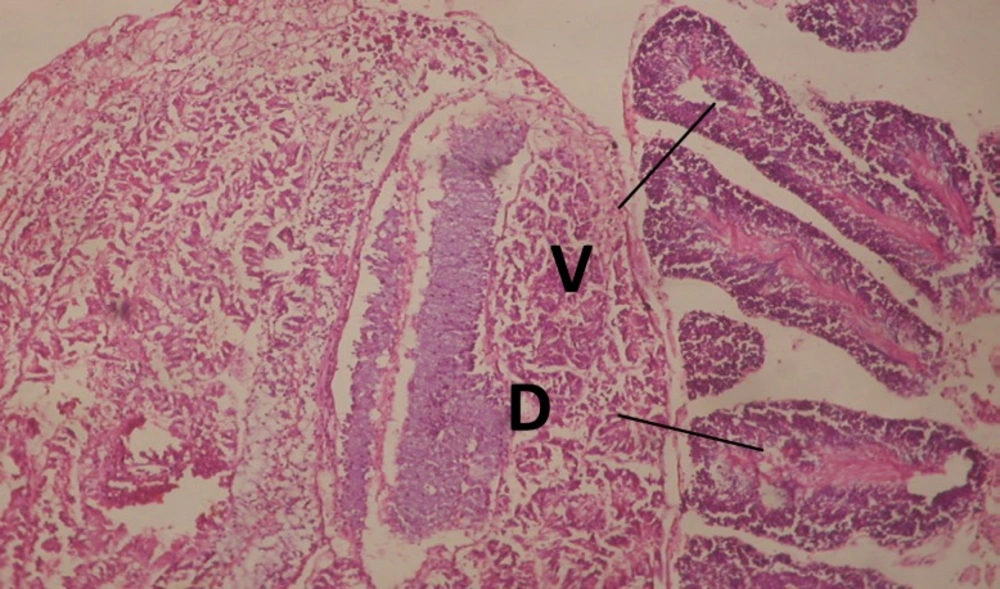
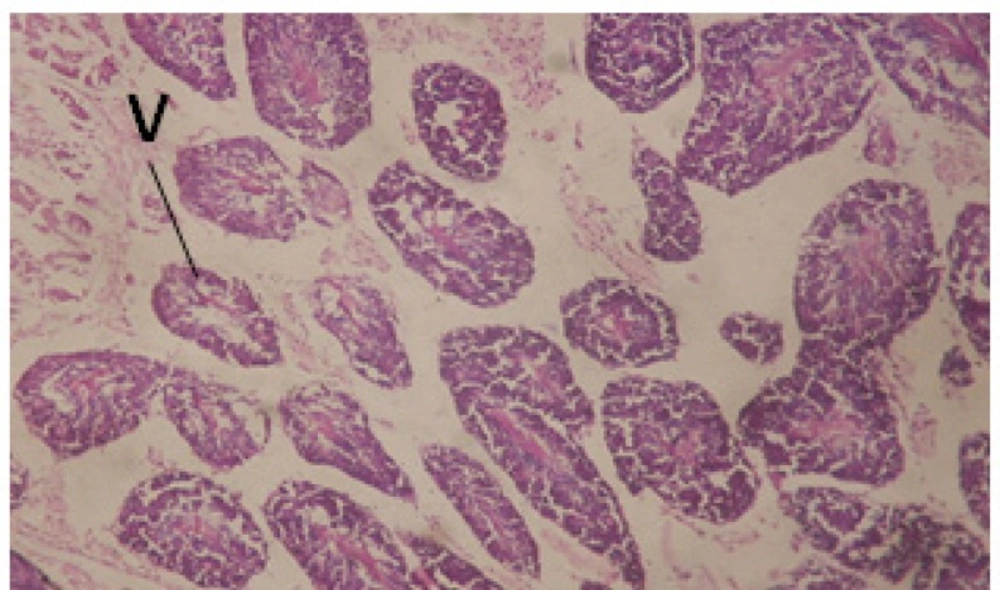
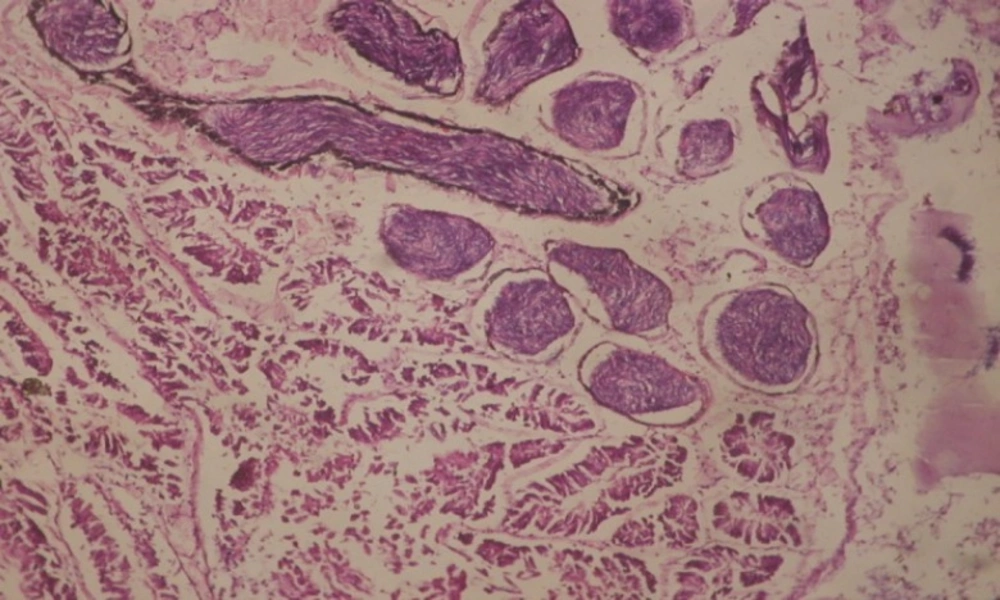
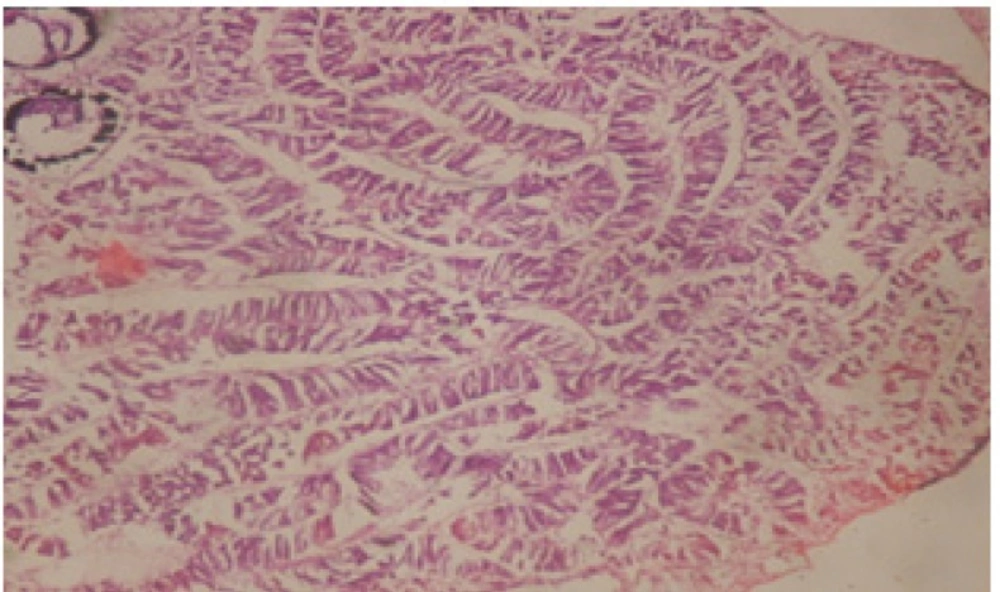
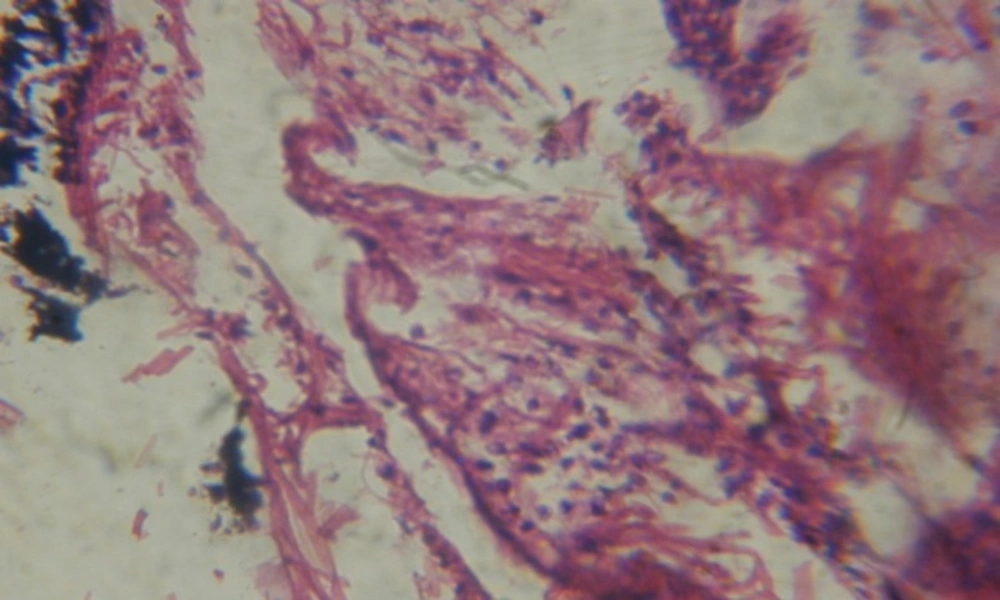
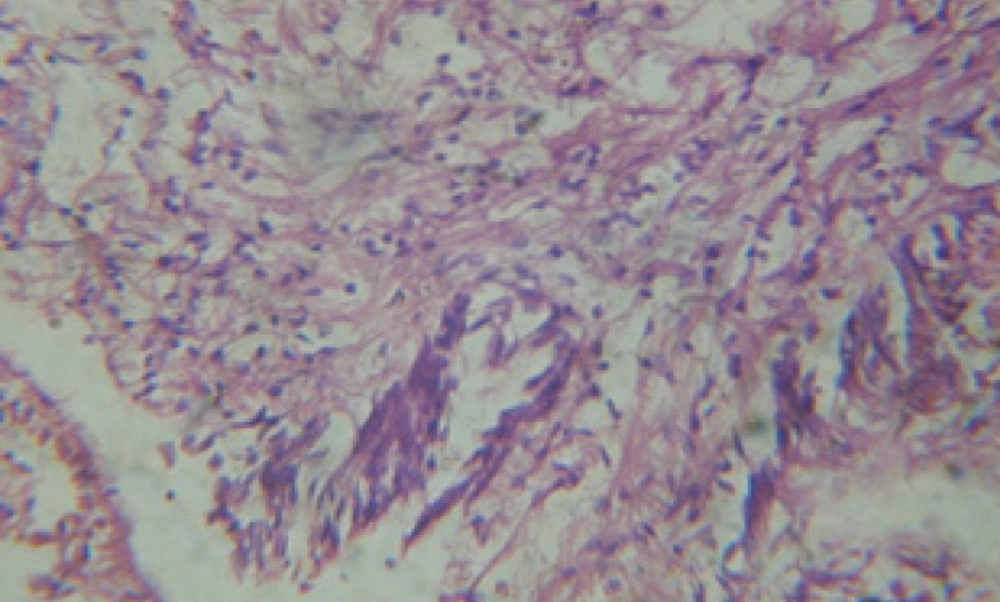
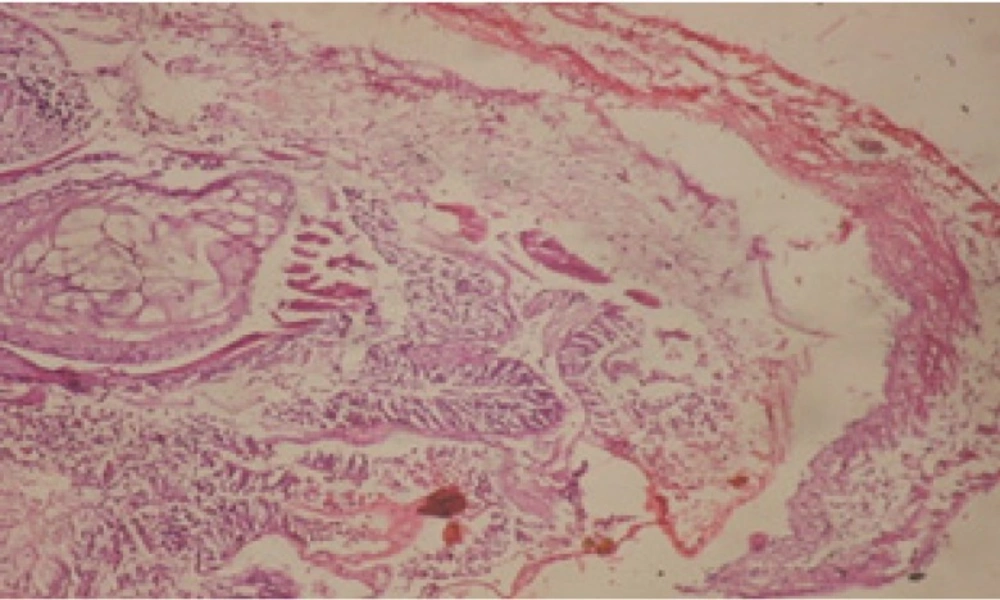
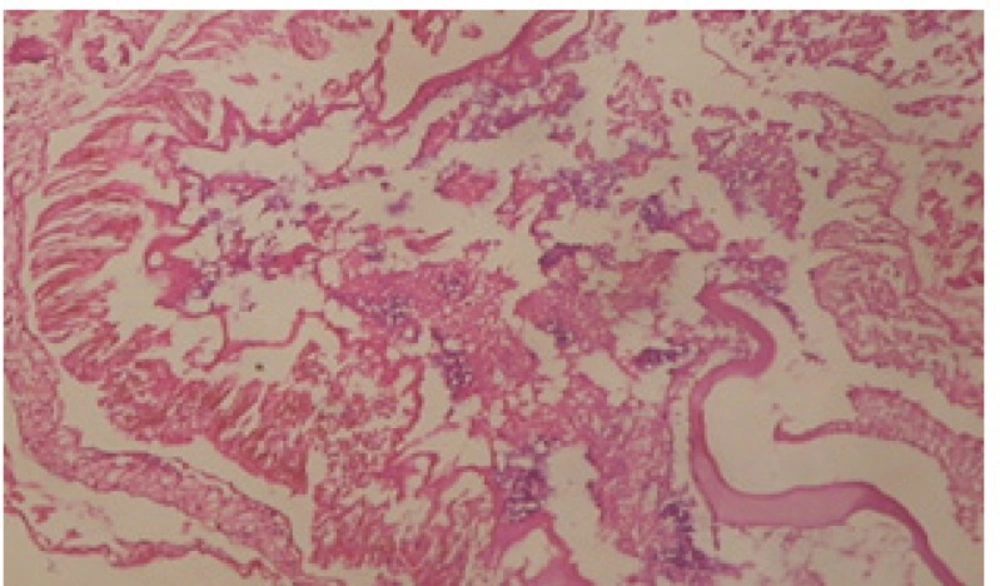
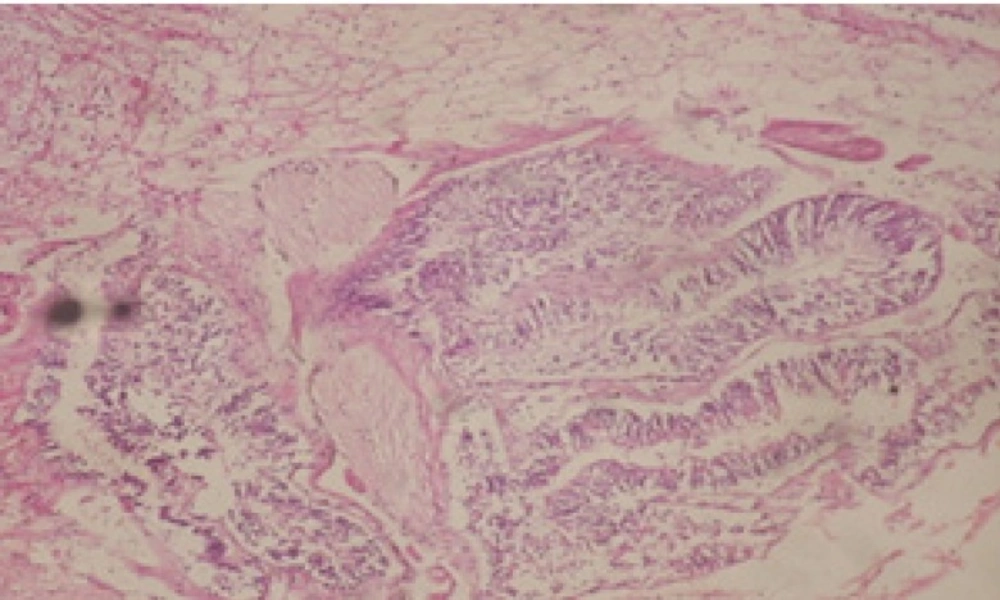
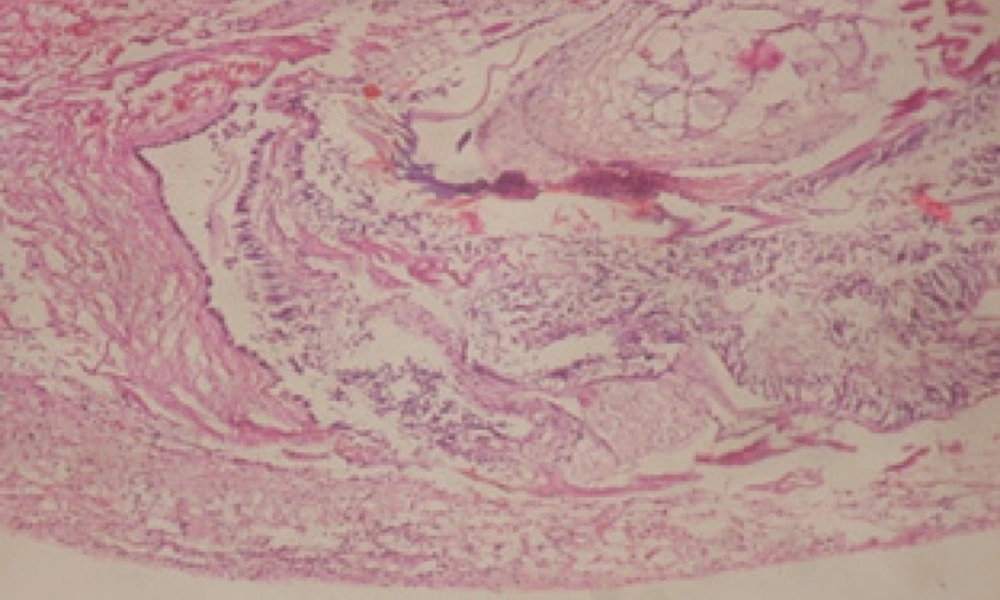
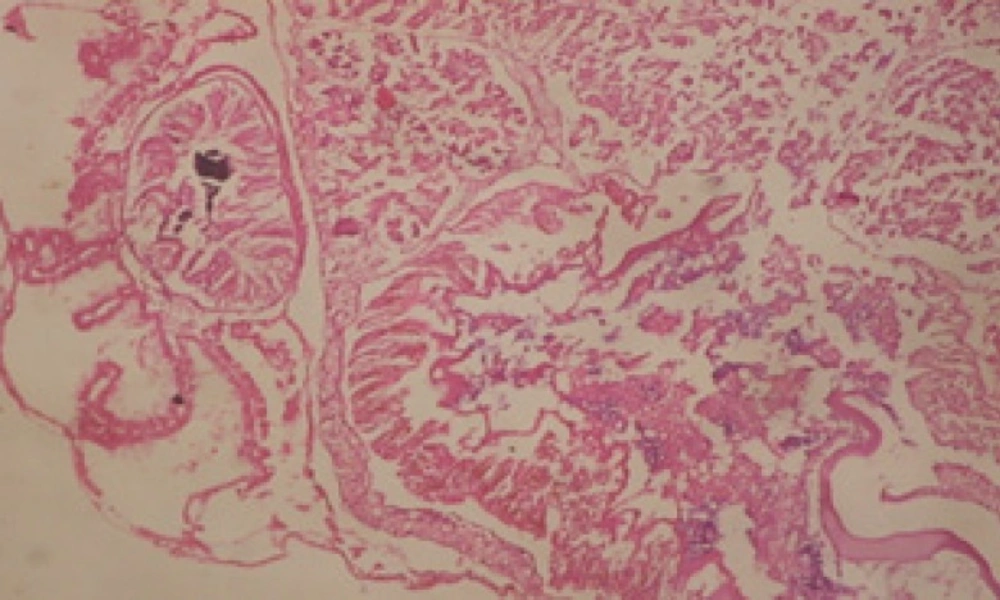
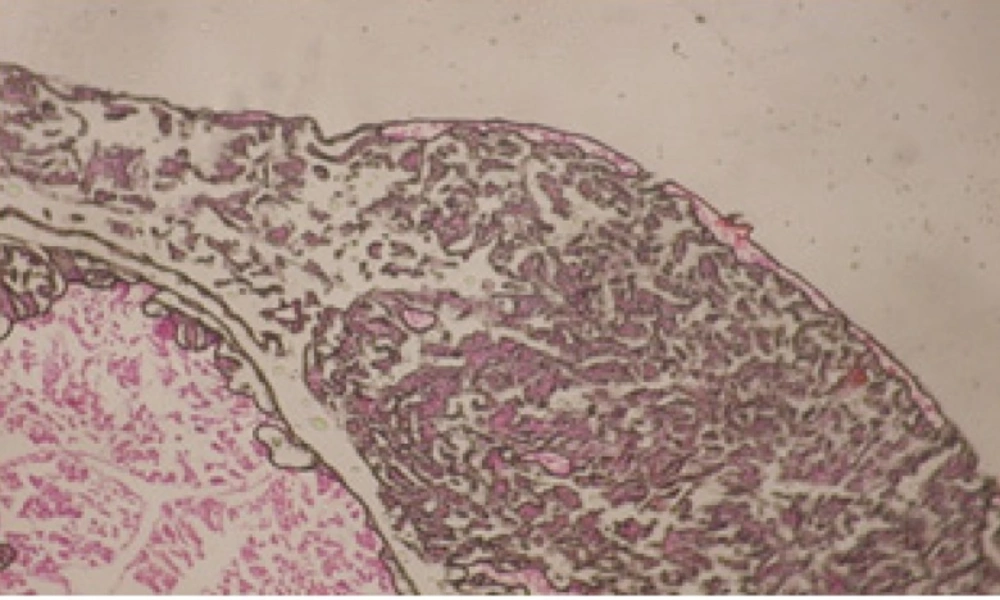
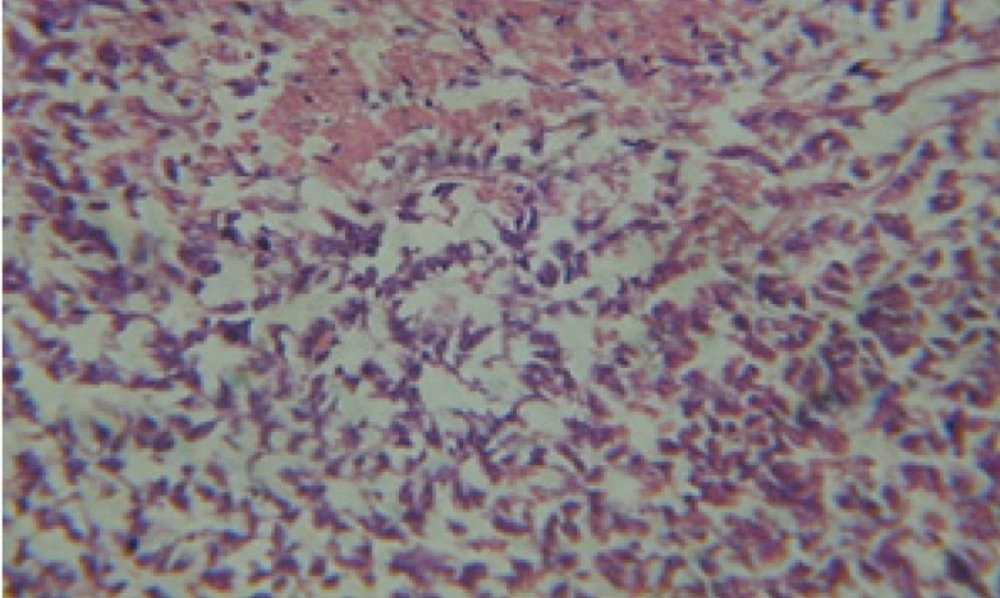
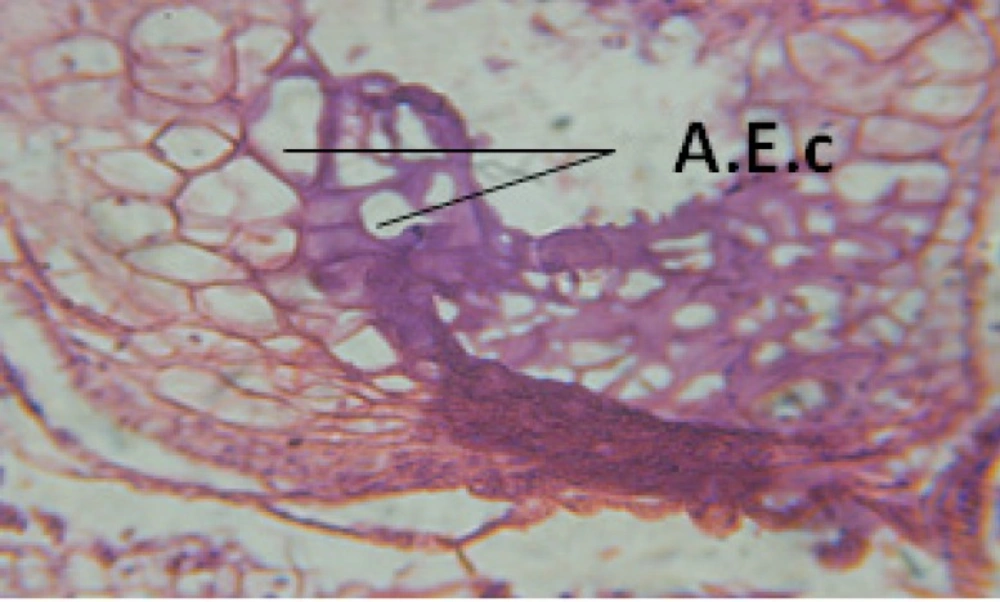
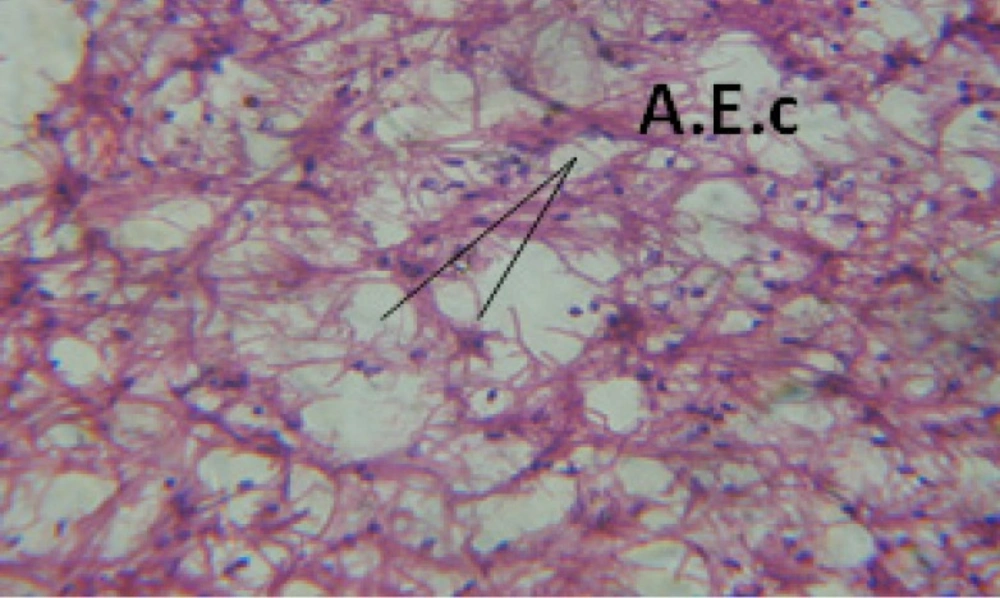
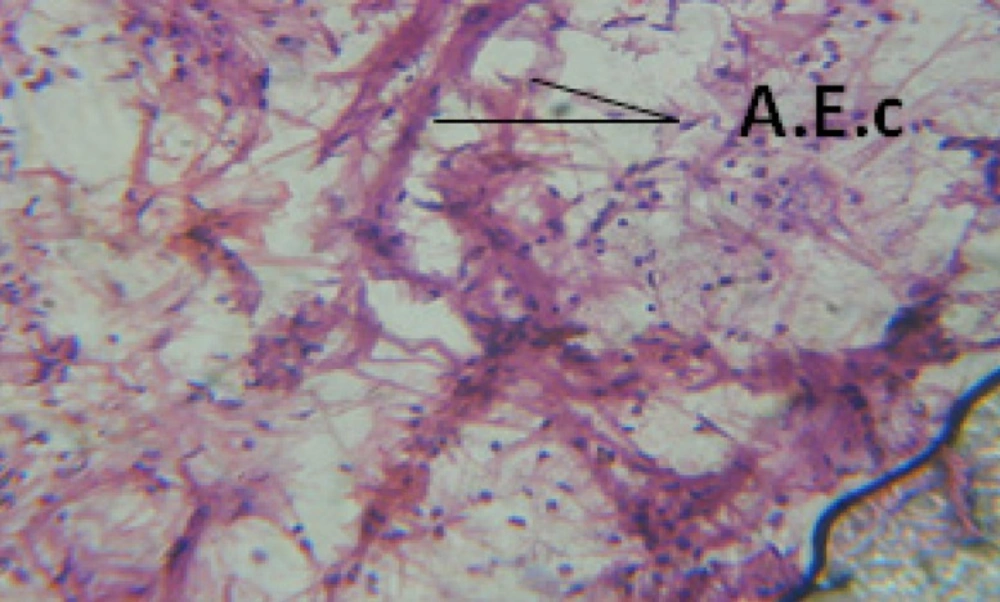
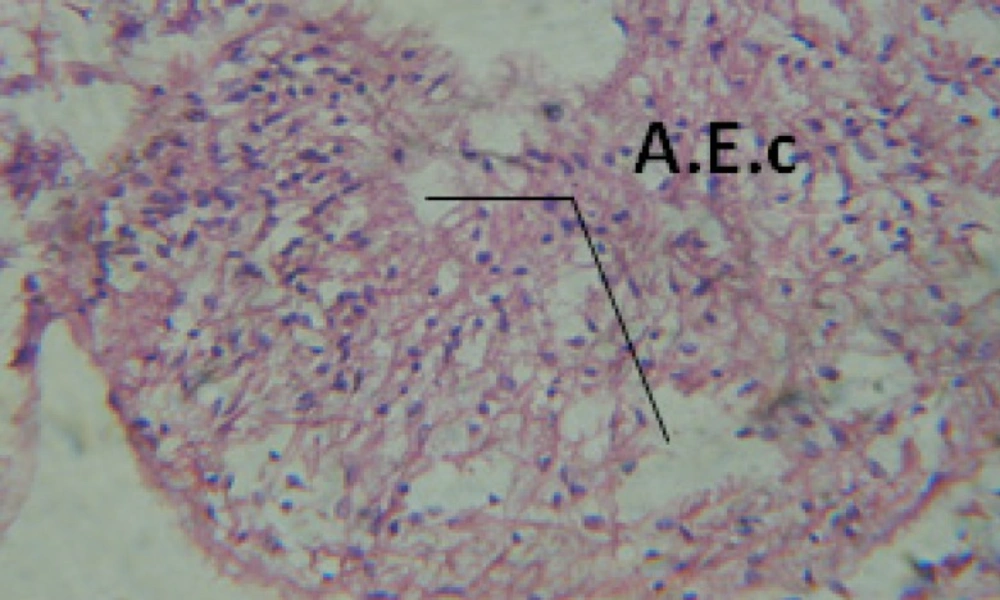
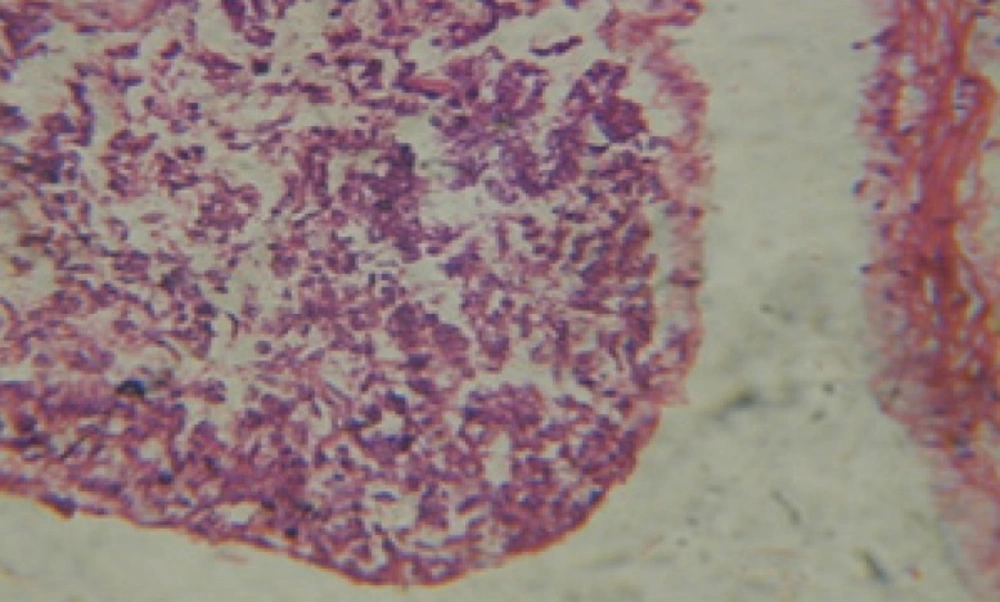
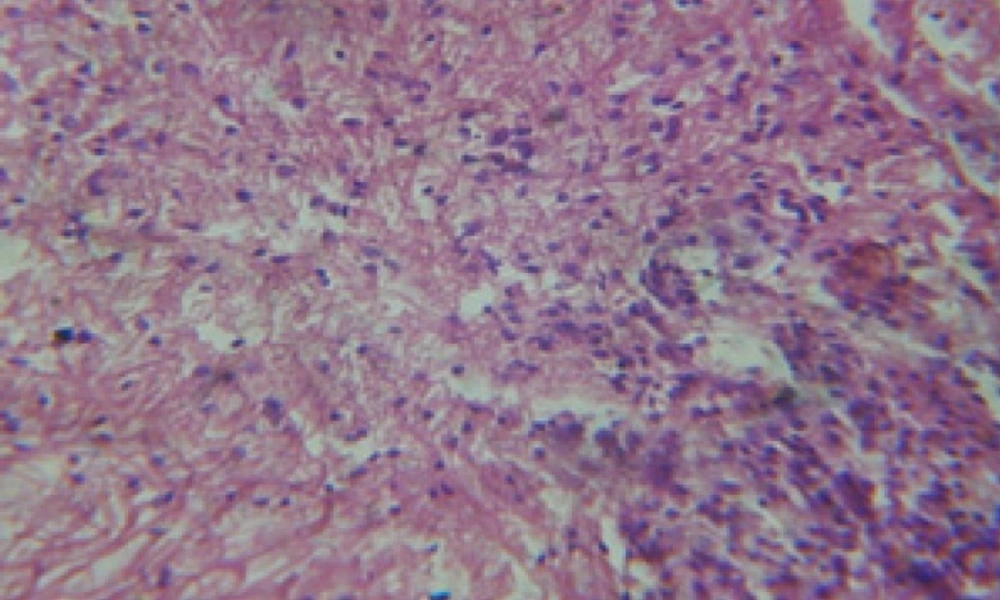
Treatment of snails with C. viminalis fruits, leaves and bark extracts showed great histopathological signs to the hermaphrodite gland and the digestive tract of the snails. The harmful histopathological changes were a function of each extract concentrations. Treated B. alexandrina with a concentration 40 ppm of C. viminalis fruits extract showed large vacuoles and degeneration in the hermaphrodite gland, destruction in the follicular membrane and the mature ovum showed losing of the nucleolus (Figure 2).
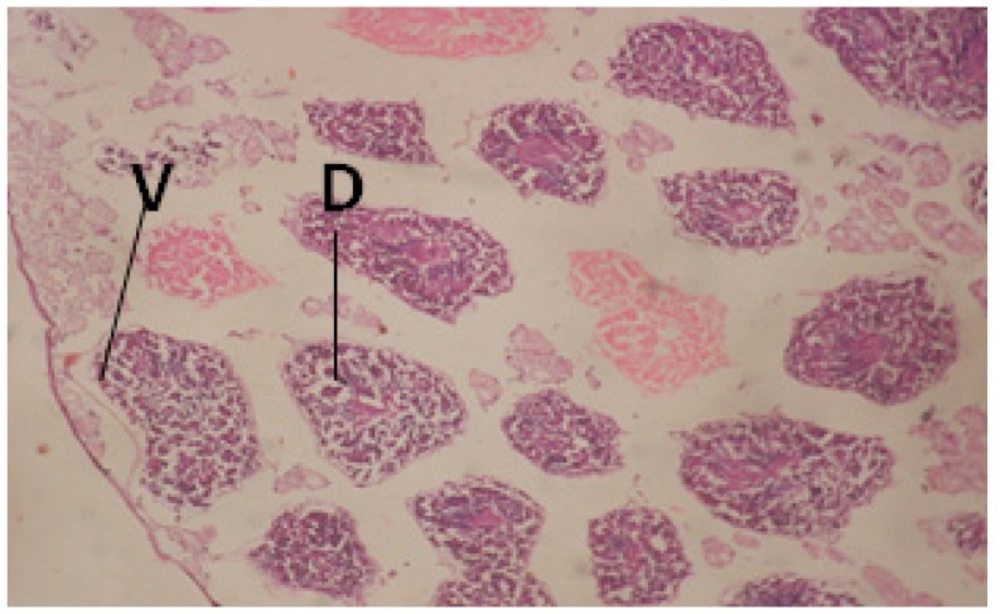
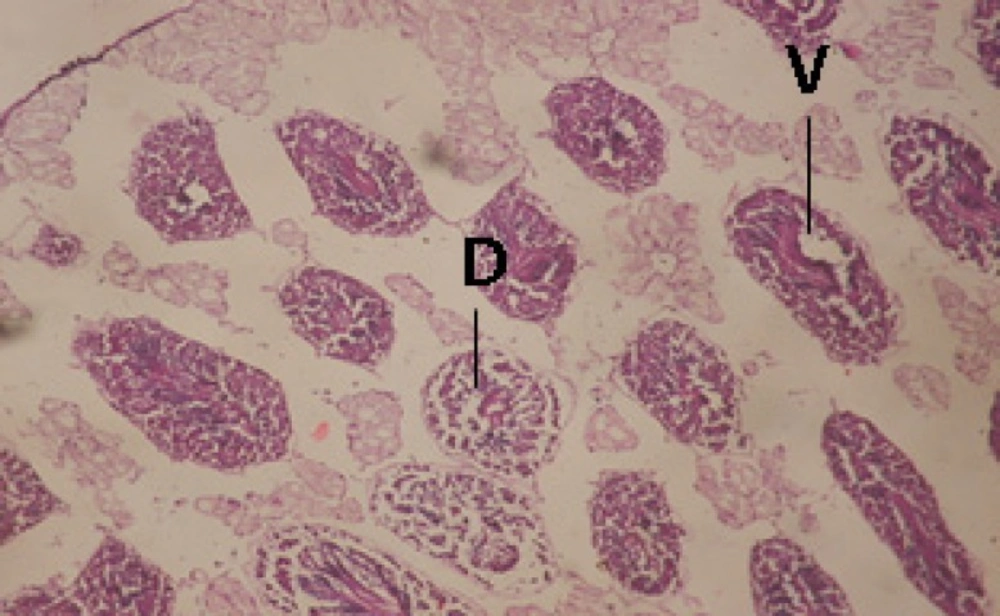
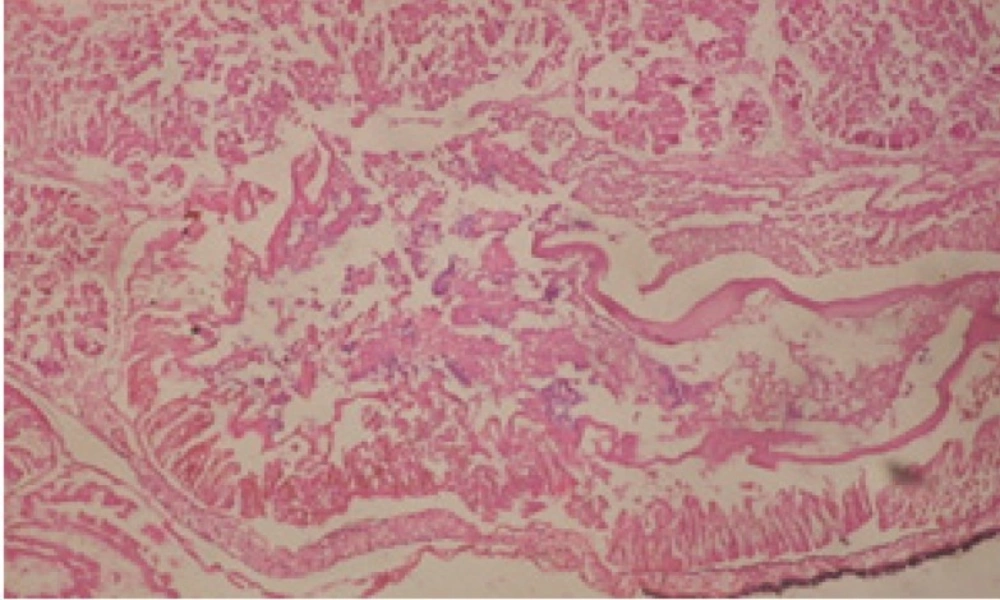
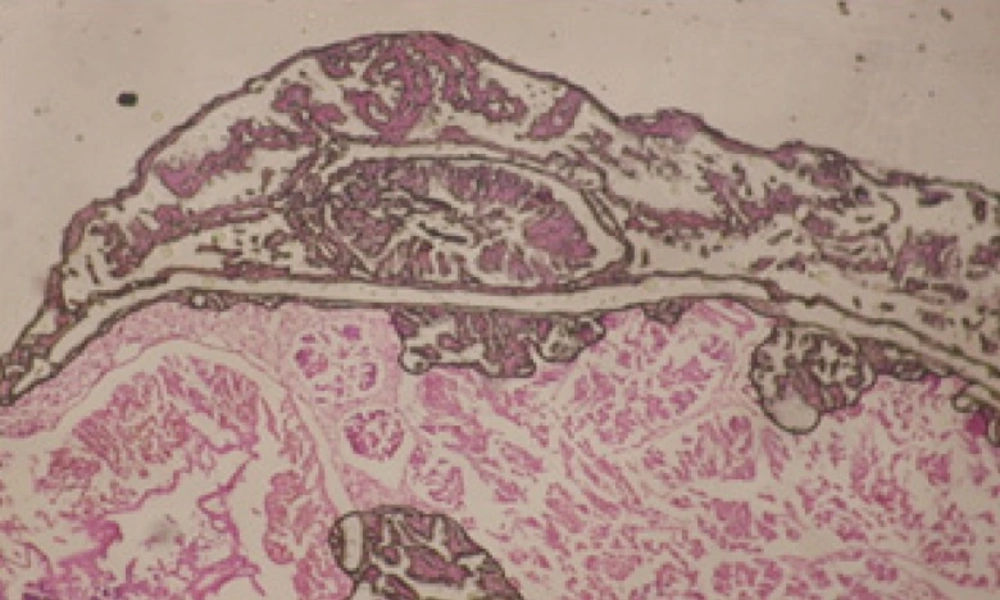
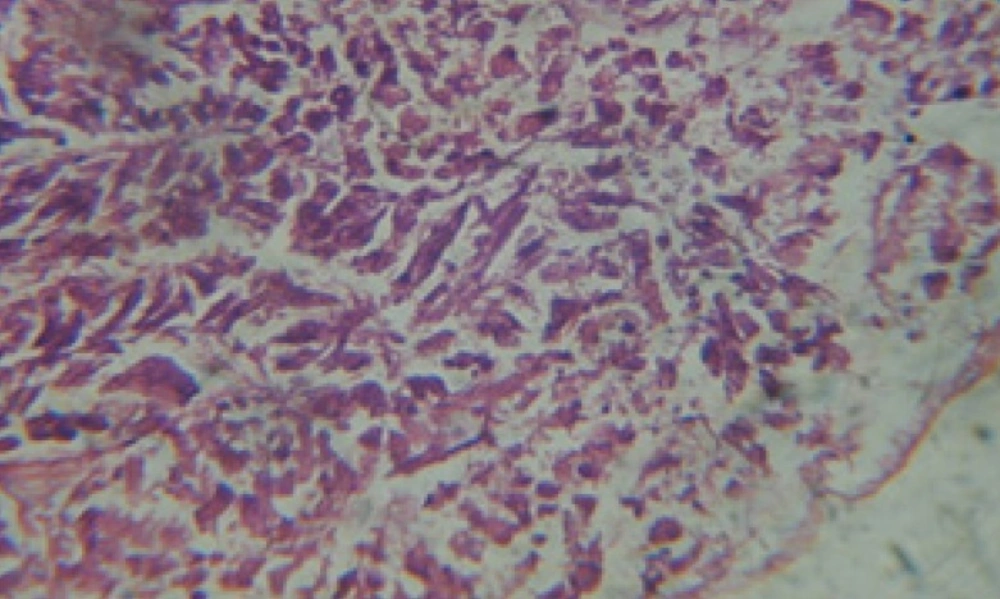
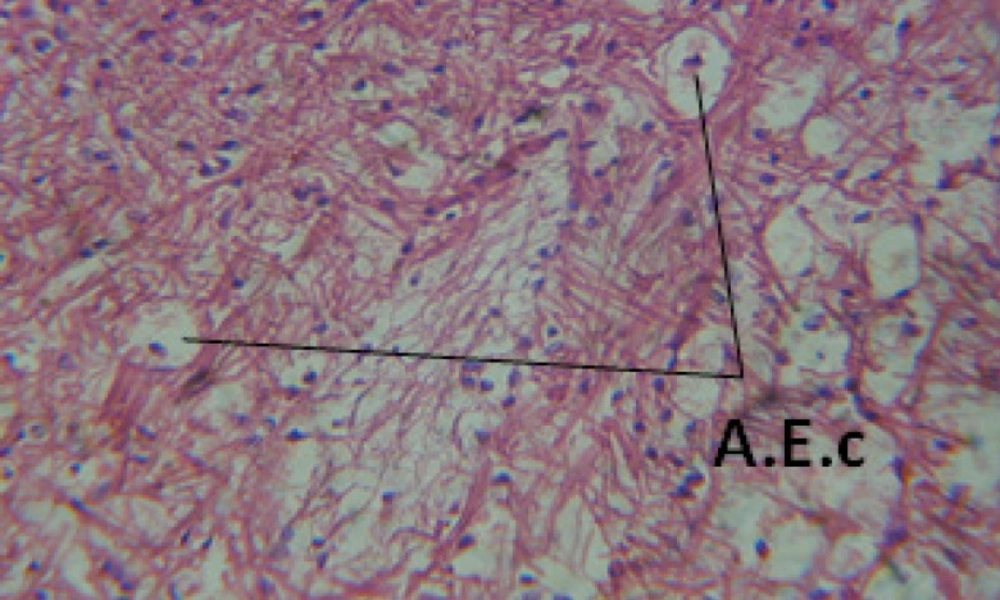
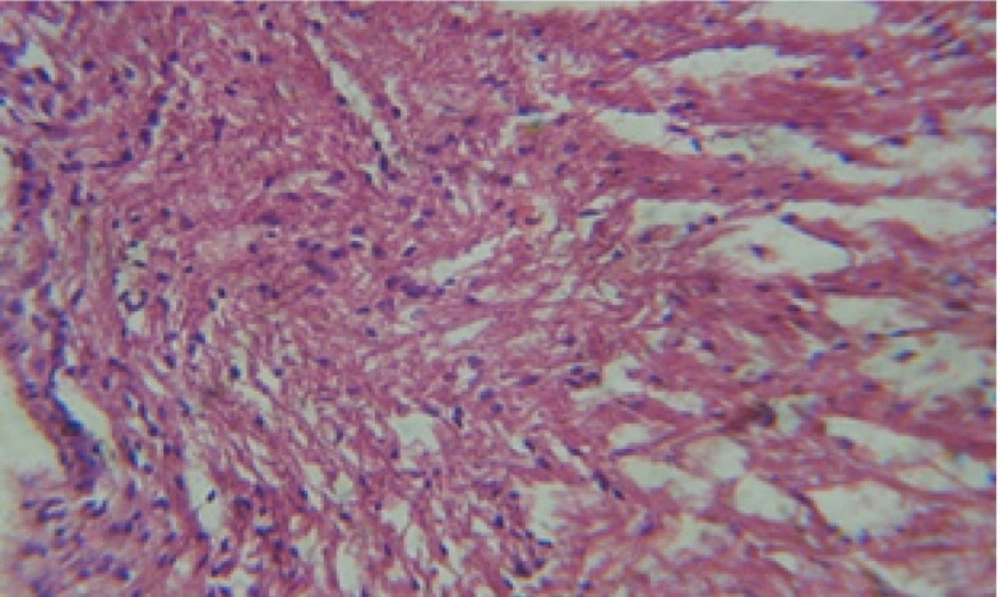
Large vacuoles and great destruction was observed in the digestive acini and the columnar epithelial cells (Figure 13). In the digestive epithelia, there observed large evacuated epithelial cells (Figure 24). While treatment with concentrations 20 and 10 ppm of C. viminalis fruits extract showed small vacuoles and little degeneration in the hermaphrodite gland (Figure 3 and 4). The size of vacuoles was not affected by the decreasing in concentrations of the extract and the destruction in the digestive acini and the columnar epithelial cells was less affected (Figure 14 and 15). The evacuation of the epithelial cells in the digestive epithelia was moderate (Figure 25 and 26). On the other hand, treatment with concentration 5 ppm of C. viminalis fruits extract showed small vacuoles in the hermaphrodite gland (Figure 5), normal digestive acini and normal columnar epithelial cells (Figure 16) and normal digestive epithelia (Figure 27). This indicates that mild toxicity afforded with the low concentration of the fruit extract. Treated B. alexandrina with a concentration 40 ppm of both C. viminalis bark and leaves extracts showed small vacuoles and moderate degeneration in the hermaphrodite gland (Figures 6 and 9), small vacuoles in the digestive acini and the columnar epithelial cells (Figures 17 and 20) and small evacuated epithelial cells in the digestive epithelia (Figures 28 and 31) while treatment with concentrations 20 and 10 ppm of both C. viminalis bark and leaves extracts was comparable with that of 5 ppm of the fruits extract; small vacuoles in the hermaphrodite gland (Figures 7, 8, 10 and 11), normal digestive acini and normal columnar epithelial cells (Figures 18, 19, 21 and 22) and normal digestive epithelia (Figures 29, 30, 32 and 33).
The tested plant extracts proved positive molluscicidal activity against B. alexandrina snails. It seems that the target tissues, for the tested extracts, were the hermaphrodite gland and the digestive tract. Destruction of the epithelial layer, vaculation and degeneration of secretory cells are the histopathological signs detected after treatment with the tested extracts.