Introduction
WHO has declared leishmaniasis as one of most serious parasitic diseases and a public health problem in many countries worldwide (1, 2). It is responsible for high morbidity and mortality in developing countries and the number of human cases have increased during the past decade (3, 4). On the other hand, dogs are the principle reservoirs for L. infantum, the causative agent of visceral leishmaniasis (VL) in most countries, and play an important role in the transmission to human being (3, 5, 6).
Over 50 years, pentavalent antimonials have remained the mainstay compounds used for treating both human visceral leishmaniasis (HVL) and CVL in most parts of the world (7, 8). Although there are many agents for treatment of infected dogs such as amphotericin B, antimonials, aminosidine and allopurinol, definitive curative treatment is not exist. The treatment options currently available are limited and the susceptibility of Leishmania parasites decrease progressively (9).
In human, pentamidine is mainly used for HVL treatment but its efficacy is questionable in comparison with other agents (10, 11). The first oral agent used for the treatment of HVL and zoonotic visceral leishmaniasis (ZVL) is miltefosine (8, 11, 12). Paromomycin and sitamaquine were effective and well tolerated for leishmaniasis treatment (8, 11, 13, 14).
Many researchers hint to some new synthetic components such as BTB06237, derivatives of thiadiazole and new series of quinoline tripartite hybrids from chloroquine & ethambutol (8, 15-17).
Many in-vitro & in-vivo studies and experimental/clinical trials have confirmed the immune-chemotherapy cohort’s advantages (4, 18-24).
Virtually no treatment will completely eliminate parasites from the infected animal, and even if temporary clinical improvement is achieved a relapse is to be expected weeks to years after drug withdrawal (9).
Herbs are potential sources for anti-protozoan drugs (8, 25). The biological activity of herbal crude extracts comes from alkaloids, flavonoids, phenylpropanoids, steroids and terpenoids in Aloeaceae family, Annonaceae family, Apocynaceae family & etc (8, 25).
In the literature, there are many works by researchers and research institutes around the world to discover natural compounds with anti-Leishmania activities (8, 11, 25, 26). In this regard, IMOD is a herbal mixture of Rosa canina, Urtica dioica, and Tanacetum vulgare in addition to selenium. IMOD has been patented in Europe for its potential in decreasing oxidative stress, reduction of tumor necrosis factor activity, improving T helper lymphocytes in HIV positive patients, effectiveness in experimental models of immunoinflammatory-based diseases and reduction of patient’s mortality rate in intensive care unit (ICU) without any mutagenic and genotoxic effects (27). IMOD has been well tolerated without any adverse effects in human & animals (28-38).
Although in recent years many research programs have been focused on anti-Leishmania agents but most of these regimens had limitations such as toxicity, cost, long-term therapy, availability, adverse effects and developing parasite resistance (8, 9, 39). Therefore, identifying a safe and effective alternative treatment for CVL is the main goal of this preliminary trial.
Experimental
Animals
Twenty healthy mongrel dogs (males and females) aging from 1 to 4 years were selected for this trial. Dogs were kept in individual cages in small animal hospital, Faculty of Veterinary Medicine, University of Tehran. After one month adapting period, all dogs were examined, vaccinated against common diseases (DH2PPil and Rabvac-1, Fort Dodge Animal Health, USA) and treated with the anthelmintic drugs. All dogs were serologically tested to be free of anti-Leishmania antibody by direct agglutination test (DAT) (4). Hematologic and biochemical evaluations were normal in all dogs.
Inoculation protocol
Amastigotes in compare with promastigotes seem to be more effective in inducing experimental infection and producing significant clinical symptoms at shorter period (37). So, we used a naturally infected poly-symptomatic dog from a well-known endemic area, Meshkin-Shahr district, in northwest of Iran (20).
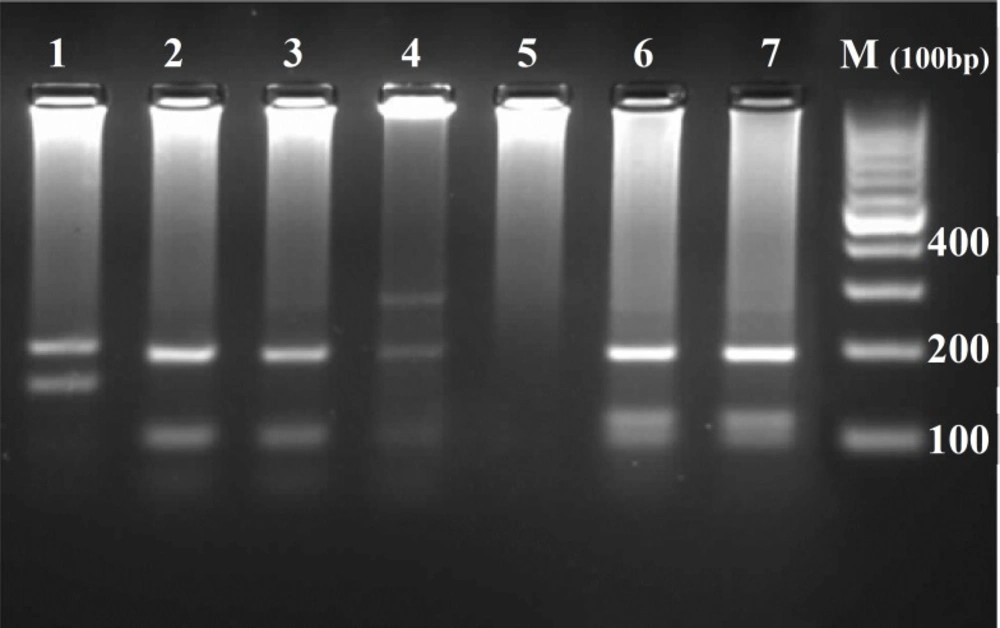
Donor dog was diagnosed by clinical examination and rK39 dipstick (DiaMed-IT LEISH, Belgium) (40). Definitive diagnosis achieved by serological screening (DAT) followed by bone marrow puncture and parasitological confirmation. Isolated parasite was identified as L. Infantum by PCR-RFLP technique using primers derived ITS1 gene and HaeIII restricted enzyme (Figure 1) (41).
The spleen of donor dog was removed by surgical laparoscopy procedure in aseptic condition and in the same manner of other authors (6, 20); then the spleen chopped to small pieces, macerated in normal saline solution in a grinder and diluted to provide desired amastigote count; 3-5 mL (based on body weight of dogs) of the spleen homogenate containing at least 107 amastigotes/mL was injected intravenously to 16/20 dogs.
Study design
Dogs were randomly divided into five groups with four animals each and were treated using the following protocols: (a) group I: negative control as an environment control and did not receive any injection; (b) group II: received Glucantime® (Sanofi Aventis pharmaceutical Co., France) 100 mg/Kg/day, for 30 days consecutively (42); (c) group III: received Glucantime® in the same dosage as group II plus infusion of IMOD (Rose Pharmed Biotechnology Co., Tehran, Iran), 2 mg/Kg with 100 mL DW 5% over 1 hour, every other day for one month (based on manufacturer recommendation); (d) group IV: received infusion of IMOD in the same dosage as group III; and (e) group V: positive control which received sterile normal saline (Table 1).
| Groups | Sex | Body weight (Kg) | Age (year) | |
|---|---|---|---|---|
| male | Female | |||
| Negative Control | 2 | 2 | 15.70 ± 0.66 | 1.50 ± 0.50 |
| Glucantime® | 2 | 2 | 18.02 ± 1.63 | 2.37 ± 0.31 |
| Glucantime®+IMOD | 1 | 3 | 17.50 ± 0.89 | 2.12 ± 0.31 |
| IMOD | 2 | 2 | 17.00 ± 1.29 | 3.37 ± 0.37 |
| Positive Control | 3 | 1 | 18.22 ± 1.15 | 3.25 ± 0.47 |
The base line characteristics in different groups of dogs (data represented as Mean±SE).
Animals were monitored regularly by clinical examination, hematological and serological tests on days 30, 60, 90 and 120 after the inoculation. For confirmation of parasite establishment and subsequent Leishmania infection, bone marrow punctures were done. The mentioned samples underwent Giemsa staining followed by light microscope observation for parasite detection.
Treatment protocols were instituted 120 days after inoculation and confirming Leishmania infection. The animals were clinically examined each day for monitoring any complications during the treatment period. Clinical signs associated with CVL were evaluated and monitored at monthly intervals up to 90 days after the end of treatment. A complete blood cell count and biochemistry profile (liver, kidney and electrolytes) were done for all dogs. DAT was done and anti-Leishmania antibody titers were considered positive at ≥1:320 (4). For evaluating the spleen size, ultrasonography was performed before and after treatment periods.
At the end of the experiment, all the animals were anesthetized and bone marrow and spleen biopsies were obtained for parasitological evaluation (43-45). Using an i.v. overdose of barbiturate, they were euthanized and necropsied. Liver and spleen samples of all dogs were taken for histopathological evaluation.
IMOD
In brief, IMOD (Rose Pharmed Biotechnology Co., Tehran, Iran) is the herbal mixture of Rosa canina, Urtica dioica, Tanacetum vulgare and selenium exposed to a pulsed electromagnetic field and dispensed into sterile ampoules for research use. Each ampoule contains 125mg of active ingredients in 4 mL solvent (27).
Ethical approval
The trial was reviewed and approved by the Ethical Committee of University of Tehran and conducted according to the Principles of Laboratory Animal Care.
Statistical analysis
All data were analyzed with t-test in pre-inoculation and pre-treatment periods within each group. Repeated measure one-way ANOVA and Tukey multiple comparison post-hoc tests were performed between groups in different times. The influence of different treatment protocols on clinical signs was evaluated using descriptive analysis such as McNemar and Fisher’s exact tests. Differences between groups were considered significant for P < 0.05. All the analyses were performed by SPSS ver.16 (Chicago, IL, USA).
Results
Clinical manifestations
In this experimental study, the most common clinical post-inoculation manifestations were weight loss (16/16), lymphadenopathy (15/16), pneumonia (13/16) and lethargy (8/16). Skin lesions (1/16) and hemorrhagic rhinitis (1/16) were detected as lowest clinical signs.
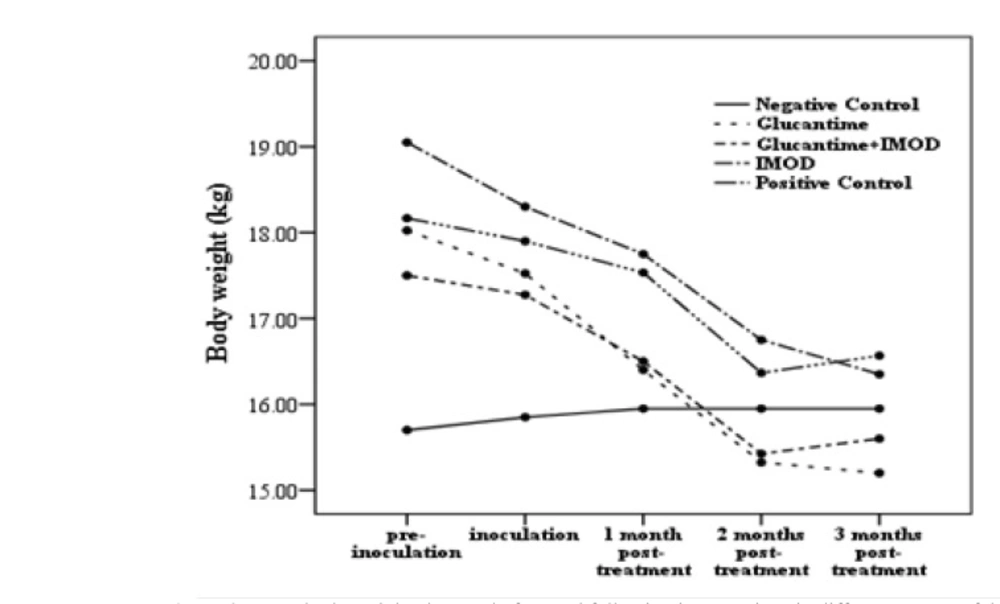
In contrast to the group II, lymphadenopathy resolved 30 days after treatment in groups III & IV. Pneumonia was deteriorated in groups II & IV and resolved in group III (immune-chemotherapy) 30 days after treatment. In animals treated by immune-chemotherapy, lethargy disappeared gradually after 30 days whereas this finding was not detected in groups II & IV. One of the lowest clinical sign frequencies (3/16) was nasal mucopurulent discharge which showed no significant difference between pre- and post-treatment periods. Despite medical intervention, skin lesions and asymmetric alopecia (2/16) did not resolve in treated dogs and showed progressive trend. Based on statistical analysis lymphadenopathy, pneumonia and lethargy showed no significant differences (P=0.08). 60 days post-treatment, weight loss had shown significant difference (P=0.02) in group III (Figure 2).
Hematology
In post-inoculation period, the significant hematological changes in all groups were increasing red blood cell distribution width (RDW), decreasing packed cell volume (PCV), neutropenia, monocytosis and lymphocytosis.
30 days after treatment, hematologic changes showed significant difference (P=0.02) between group IV and group V. These differences were not significant 60 days after treatment in mentioned groups (IV&V) which may be due to deterioration of clinical signs. In groups II & III, hematological profiles showed no significant differences during the treatment periods. All the hematological parameters were reduced in control positive animals until the end of study.
Blood chemistry
Based on biochemistry profile analyses, significant elevation of blood glucose and triglyceride were detected in infected dogs after inoculation. Blood urea nitrogen (BUN), albumin, Mg2+ and Cl- also showed significant reduction in infected dogs.
In all treated groups, total protein, albumin, BUN and liver enzymes (ALT & AST) did not show any significant differences between inoculation and treatment periods. In IMOD (IV) group, cholesterol, HDL & LDL showed significant differences (P=0.01, 0.02, 0.02 respectively) 30 days after treatment but these findings did not remain until the end of study. 30 days after treatment, serum calcium level showed statistically significant difference (P=0.009) between group I & group II. 60 days following the treatment, changes in phosphorus level were not significant but after this time, significant reduction trend (P=0.02) was detected in IMOD group. At the end of the study (90 days after treatment), there was significant difference between groups II and V in serum Cl- level. Comparing with control positive group, a significant reduction (P=0.02) in serum Na+ level were shown in groups III & IV, 60 days after treatment. Differences for the other serum electrolytes such as Fe2+, Mg2+ and K+ were not noticeable during treatment periods.
Serology
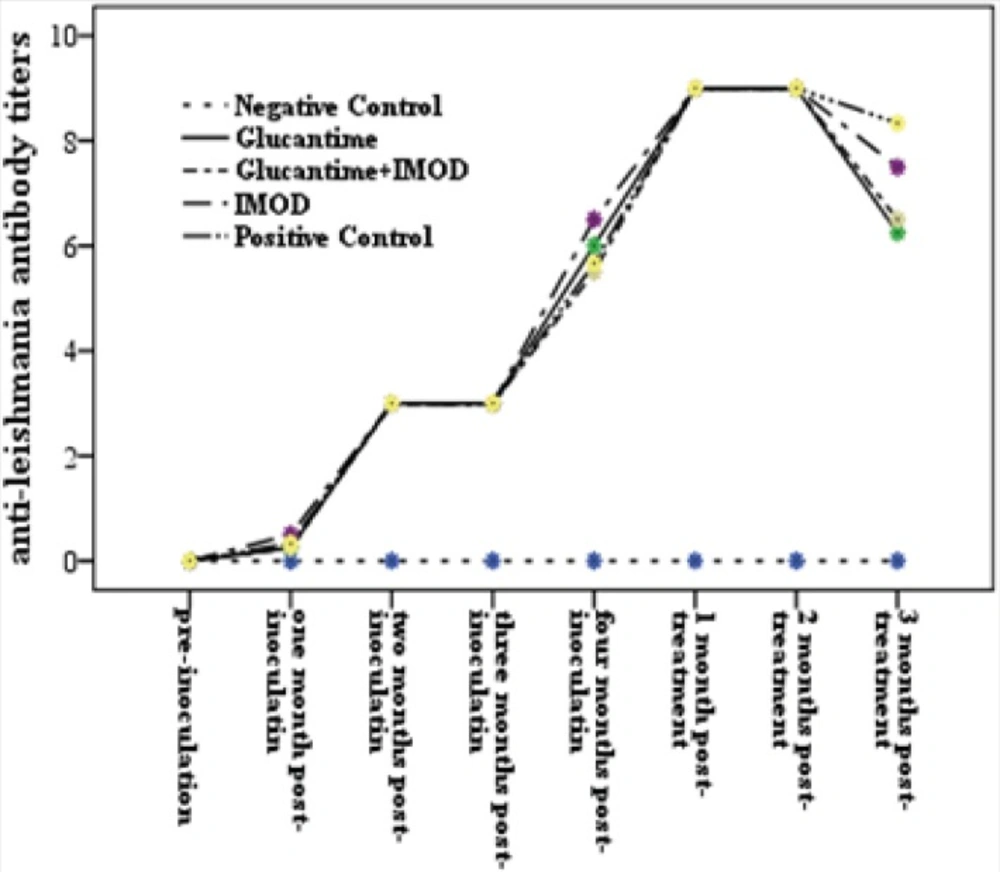
Based on serological results, all dogs had positive titers (≥1:320) 60 days following inoculation.
In this experiment 60 days after treatment, a number of dogs in groups II (chemotherapy), III (immune-chemotherapy) and IV (immunotherapy) showed reduction of anti-Leishmania antibody titers (4/4, 3/4 and 2/4 dogs, respectively). In all treated groups, decreasing of DAT titers were significant 60 days after treatment, however none of them became seronegtive (<1:320) (Figure 3).
Imaging technique
In this study, to evaluate the effects of various treatments on possible splenomegaly and reducing parasitic load in pre-/post- treatment periods, the spleen sizes of all dogs were measured by ultrasound device (Vivid7, General Electric, USA). 30 days after treatment, a significant differences (P=0.046) in spleen sizes were detected between groups III & V. The differences of spleen size between group IV and group V were significant (P=0.02) 60 days after treatment, as well.
Parasitology
To confirm definitive infection of dogs, 120 days post-inoculation, parasitological evaluation of bone marrow punctures was performed. At this stage, amastigotes were detected in 12/16 bone marrow aspirations by light microscopy and remaining samples were confirmed by parasite culture.
The spleen biopsies remained positive in treated animals at the end of experiment, except one dog (1/4) in group II and one (1/4) in group III, which became negative for Leishman bodies. Negative results of these dogs were confirmed by conventional PCR method (46).
Histopathology
Formalin-fixed spleen & liver samples were processed through paraffin embedding, sectioned at 4 µm with a rotary microtome, placed on a glass slide, stained with Hematoxylin & Eosin and evaluated microscopically. In this experiment, histopathological findings for liver samples were as follows: mixed cell inflammation, peritubular and bile duct fibrosis which were not significantly different among control (I & V) and treated (II, III & IV) groups.
In group II, except in one dog (1/4) with local hemorrhage, marginal zone hyperplasia was detected in spleen tissue sections (3/4). Marginal zone & follicular hyperplasia were observed in all dogs treated by immune-chemotherapy (4/4). In groups I, IV and V marginal zone hyperplasia were evaluated in spleen sections as well (Table 2).
| Groups | Spleen | Liver | PF | BDF | ||
|---|---|---|---|---|---|---|
| MZH | FH | LH | MCI | |||
| Negative Control | 4/4٭ | 0/4 | 0/4 | 4/4 | 4/4 | 4/4 |
| Glucantime® | ¾ | 0/4 | 1/4 | 4/4 | 4/4 | 4/4 |
| Glucantime®+IMOD | 4/4 | 4/4 | 0/4 | 4/4 | 4/4 | 4/4 |
| IMOD | 4/4 | 0/4 | 0/4 | 4/4 | 4/4 | 4/4 |
| Positive Control | 4/4 | 0/4 | 0/4 | 4/4 | 4/4 | 4/4 |
Frequencies of histopathological changes of liver and spleen in treated and control dogs at the end of study.
Discussion
Canine visceral leishmaniasis (CVL) caused by Leishmania infantum is the main reservoir for human infection which is a zoonosis potentially fatal to humans and dogs (47). So far, no agents have shown potency for complete elimination of parasites. Induction of parasite resistance, lack of parasitological clearance, expense and toxic effects caused limitations of conventional therapies progressively (9). Although many promising agents have been described recently, few have acceptable effects. The host’s immune system plays a fundamental role in the establishment of infection and in therapeutic response. So, further effective and safe alternative anti-Leishmania or potential immunomodulator agents in addition to conventional therapeutic regimens seem necessary. This is the first trial evaluating a novel herbal medicine, IMOD, for treating experimental CL.
Based on Chapman et al. (44), spleen of dog and hamster is suitable organ for experimental inoculation because of higher parasite burden due to the low level of IFN-γ. So, in this experiment, we inoculated the suspension of live parasites derived from the spleen of donor dog to our dogs through i.v. injection. In this way, the natural process is accelerated and induces a stronger immunity response and more obvious clinical symptoms in dogs. For designing experimental studies, time and cost saving are the advantages of using the mentioned protocol (6, 20).
Over 50 years, the use of chemotherapy, based on pentavalent antimonials, has made them as the first step for treating VL in both human and dogs. However, due to recently development of drug resistances associated with various leishmanial species (5), the need for new agents is more emphasized.
Pentavalent antimonials inhibit two essential enzymes (phosphofructokinase and dehydrogenase pyruvate) for parasite survival (21, 48, 49) but in dogs has been mostly unsuccessful. Although major clinical signs of disease disappear after treatment, this might not indicate the complete absence of parasites in spleen or bone marrow (30). Combination of anti-Leishmania agents with vaccines and/or immunomodulator drugs might enhance drug efficacy, stimulate patient immunity and reduce the potential adverse effects of conventional treatment (7, 8, 11, 19-21).
The complex nature of CL and its clinical manifestations make its management difficult. All the infected dogs in this experiment were categorized in stage II and III, based on staging of canine VL by Leish-Vet group (47). The most prominent clinical signs in this trial as also found in naturally infected dogs were lymphadenopathy and weight loss which could be seen along with other clinical signs or clinicopathological abnormalities (20, 21, 42, 47).
Results of Glucantime® therapy (group II) showed that some clinical signs such as pneumonia were disappeared 60 days after treatment period; the other manifestations such as lymphadenopathy, lethargy and skin lesions reappeared 30 days after treatment. In group II, reduction of anti-Leishmania antibody titers were detected 60 days after treatment, however none of dogs in this group became seronegative (<1:320) at the end of study.
In group III (Glucantime® plus IMOD), although remission of clinical signs were seen 30 days after treatment followed by normal hematobiochemistry profiles and reduction of anti-Leishmania antibodies after 60 days but negative serological results were not detected by the end of study. These findings are also reported by other authors regarding different immune-chemotherapy protocols for CL (6, 8, 20-22, 24, 46).
Resistance to leishmaniasis is well known due to cell mediated immunity and the balance of the Th1 (IL-2, IFN-γ & TNF)/Th2 (IL-10 & IL-4) responses (20, 21, 24, 42). Lack of cell reactivity particularly when specific antibody titers are high is confirmed in patients with acute VL. So, a treatment that provides clinical recovery and reduces anti-body titers, at least partially, is highly desirable (21). At the end of monitoring period (day 90), one dog from groups II and one in group III were clinically treated as confirmed by the conversion of parasitological parameter and according to the clinical classification of Leish-Vet group (47); this finding was further confirmed by PCR negative results. The clinical recovery in groups II & III could be related to the use of the Glucantime® and the immune-chemotherapy protocol and higher cellular reactivity to leishmanial antigens.
Unlike group II, dogs from group III (immune-chemotherapy) showed clinical signs improvement in a shorter time (approximately 30 days) with no relapse.
In group IV, IMOD administration as an immunomodulator drug was not associated with clinical adverse effects and the evaluation of hematological and biochemical (renal and hepatic function and electrolytes) tests showed no remarkable changes. Shortly after IMOD therapy, decreasing in the spleen sizes were detected (P=0.02); this finding may be associated with parasite load reduction. However based on parasitological and PCR evaluation, the IMOD had no effect for parasite elimination in this experiment.
In agreement with Khorram Khorshid et al. (31), histopathological evaluation for liver and spleen samples showed no significant differences with control groups (Table 2).
Based on some studies, the herbs and selenium in this complex have strong anti-oxidative stress potential and immunomodulator mettalo-enzyme, respectively (29, 50-51). As reported by other authors (35), an important point that deserves further research was the observation that no adverse effects occurred in the animals with IMOD therapy. On the other hand, animals in group III (immune-chemotherapy) showed better clinical conditions and significant decreasing in spleen size (P=0.046) comparing with group II (chemotherapy).
Although, the number of animals in each group was not large enough to ensure a statistical analysis due to many problems associated with experimental studies, this was a considerable result. These results suggest that IMOD can be an important adjuvant for anti-CL therapeutic protocol that causes resolving in clinical staging. Therefore, the institution of an immune-chemotherapeutic protocol constituted of IMOD with Glucantime® may help to open new windows for treatment of leishmaniasis, consequently decreasing the clinical manifestation. This is a very interesting possibility that needs further investigation for both leishmaniasis and the other immune mediated diseases.
In conclusion, these experimental data suggest that administration of IMOD in combination with conventional treatment reduce the developing of clinical signs in shorter period and improved the quality of patients’ life. But the relatively low number of animals in each studied groups, short follow-up period and the possible presence of other differences such as individual characteristics make these data insufficient to draw any conclusion regarding the curative effect of IMOD in this disease. For confirmation of these data, more clinical and/or experimental studies and extending follow-up period after treatment with using Real-time PCR (43, 46, 47) are recommended.