Introduction
Cardiovascular diseases are becoming the main cause of mortality and morbidity throughout the world. The high prevalence of this disease has resulted in the introduction of more advanced treatment methods and drugs in recent decades (1). One of the most dangerous cardiac diseases in coronary arteries occlusion, which in some cases results in the need for surgical procedures, in the form of angioplasty or bypass surgery. In last 30 years, newer methods like stent implantation and new drug regiments have resulted in better outcomes (2). The invention of these new methods has caused some new concerns (1). Placing a stent is one of the methods used to treat the occlusion which is performed using a bare metal or a drug-eluting stent, both of which can result in thrombosis (3).
Platelets play a critical role in acute coronary syndrome (ACS) caused by thrombotic events during and after the percutaneous coronary intervention (PCI). Aspirin and clopidogrel are prophylactic drugs used in patients undergoing angioplasty to reduce the rate of thrombosis and related morbidity and mortality (3). Clopidogrel is a pro-drug which is activated by CYP450 liver enzyme (4).
The active metabolite of clopidogrel makes an irreversible bond with P2Y12 ADP receptor and stops the platelet aggregation (5). Eighty-five percent of clopidogrel is hydrolyzed to inactive carboxylic acid derivatives and 15% is oxidized into 2-oxyclopidogrel by CY450 and then turned into active thiol derivatives (6), with CYP2C19 being involved in both pathways (7).
Clopidogrel is a selective ADP-receptor antagonist that bonds to this receptor in platelet surface as a non-competitive antagonist and stops the ADP from reaching its receptor. This results in a reduction in activity of glycoprotein in platelet (GPIIb/IIIa) needed for fibrinogen-platelet adhesion (8).
The antiplatelet activity of clopidogrel is not equal in all patients. Some patients do not show a good response to clopidogrel (3) and have a higher risk of thrombosis. It has been demonstrated that this resistance is related to higher rate of cardiovascular events in these group of patients compared to nonresistant patients (3). Between 4 to 30 percent of patients treated with 75 mg/day of clopidogrel has been reported to show resistance in different studies (3). Interpersonal difference in response to clopidogrel is multifactorial and can be due to intrinsic or extrinsic mechanisms (3).
Extrinsic mechanisms can be related to low dosage in patients with a history of PCI or interference with other drugs and intrinsic factors are gene polymorphism or up regulation of other platelet activity pathways. In previous studies, clopidogrel non-responders have been defined as those patients whose platelet activity after drug usage drops less than 10% compared to their level before using drug. Patients whose platelet activity drops between 10 to 30% after the drug, are referred as semi-responder and complete response is defined as more than 30% reduction in activity compared to pre-drug state (3).
To the best of our knowledge, the prevalence of drug resistance to clopidogrel and its role in therapeutic outcome in Iran have not been investigated. The present study was performed to access the prevalence of drug resistance and the role of this resistance in patients’ outcomes among Iranian patients and methods.
Experimental
Patients and methods
The population in the present study was comprised of patients undergoing angioplasty with a stent placement in the department of cardiology, Imam Hossein Medical Center, Tehran, Iran. All patients were given a written informed consent before entering the study, and the study was performed in accordance with the declaration of Helsinki.
Inclusion criteria were angioplasty procedure with drug elating stent (DES) placement and consuming standard dosage of aspirin (75-325mg/day). The exclusion criteria were age under 18, history of clopidogrel usage in last 1 month, acute infarction in last 18 h, platelet count < 100000 mm3, hematocrit < 25%, creatinine > 4 mg/d, usage of glycoprotein IIb/IIIa before procedure, history of alcohol consumption, previous PCI, contraindications due to any cause for anticoagulant therapy, severe disease with life expectancy under 1 year and finally patients with cancer or patients undergoing dialysis. Blood sampling from patients was performed from June 2011 to June 2012. For all patients entering the study, a questionnaire including demographic information was completed.
After entering the study, patients received a loading dose of 600 mg clopidogrel (Plavix®, Sanofi, France) before the angioplasty and a daily dose of 75 mg afterward. Platelet aggregation test was performed for all patients before and 24 h after taking the loading dose of clopidogrel using light transmission aggregometry (LTA) method using a 4-channel Labtec APACT 4004 aggregometer. LTA is the gold standard method for studying the platelet activity (2). The response to drug was categorized as complete resistance (platelet aggregation decreased less than 10%), intermediate resistance (platelet aggregation decreased between 10% to 20%) and complete response (platelet aggregation decreased 30% or more). All patients were interviewed by phone 1 month after the angioplasty regarding MACE criteria including deaths, nonfatal MI, and need for urgent revascularization.
Results
Thirty-one patients with an average age of 59 ± 13 entered the study. Twelve patients were female and 19 were male. Demographic findings of patients are summarized in Table 1.
| Parameter | Value | Parameter | Value | |
|---|---|---|---|---|
| Age | 59 ± 13 | Pantoprazol | 3 (9.7%) | |
| 59 (42 to 81) | Atorvastatin | 18 (58.1%) | ||
| Gender | F | 12 (38.7%) | ||
| M | 19 (61.3%) | |||
| Major Risk Factors | ||||
| Diabetes | 6 (19.4%) | Beta Blockers | 18 (58.1%) | |
| HTN | 17 (54.8%) | Nitrates | 12 (38.7%) | |
| HLP | 3 (9.7%) | Omeprazol | 2 (6.5%) | |
| Smoking | 7 (22.6%) | |||
The average platelet aggregation among patients before and after the drug administration is demonstrated in Table 2.
| Statistics | Pre | Post | Change | Relative change (%) |
|---|---|---|---|---|
| Mean SD | 54.5 ± 18.8 | 30.4 ± 19.8 | 24.1 ± 20.1 | 41 ± 36 |
| Median (Range) | 54.7 (14.8 to 88.6) | 22.5 (3.4 to 76.7) | 23.7 (-7.3 to 60.4) | 40 (-28 to 94) |
| 95% CI | 47.6 to 61.4 | 23.1 to 37.6 | 16.8 to 31.5 | 27.9 to 54.3 |
This was 54.2 ± 188 before and was reduced to 30.4 ± 19.8 after the therapy which shows a reduction of 24.1 ± 20.1. The percentage of patients who were complete responder, semi-responder or non-responder is shown in Table 3.
| Response | N | % | 95% CI for percent* |
|---|---|---|---|
| Complete Responder | 20 | 65% | 45% to 81% |
| Semi Responder | 7 | 22% | 10% to 41% |
| Non-responder | 4 | 13% | 4% to 30% |
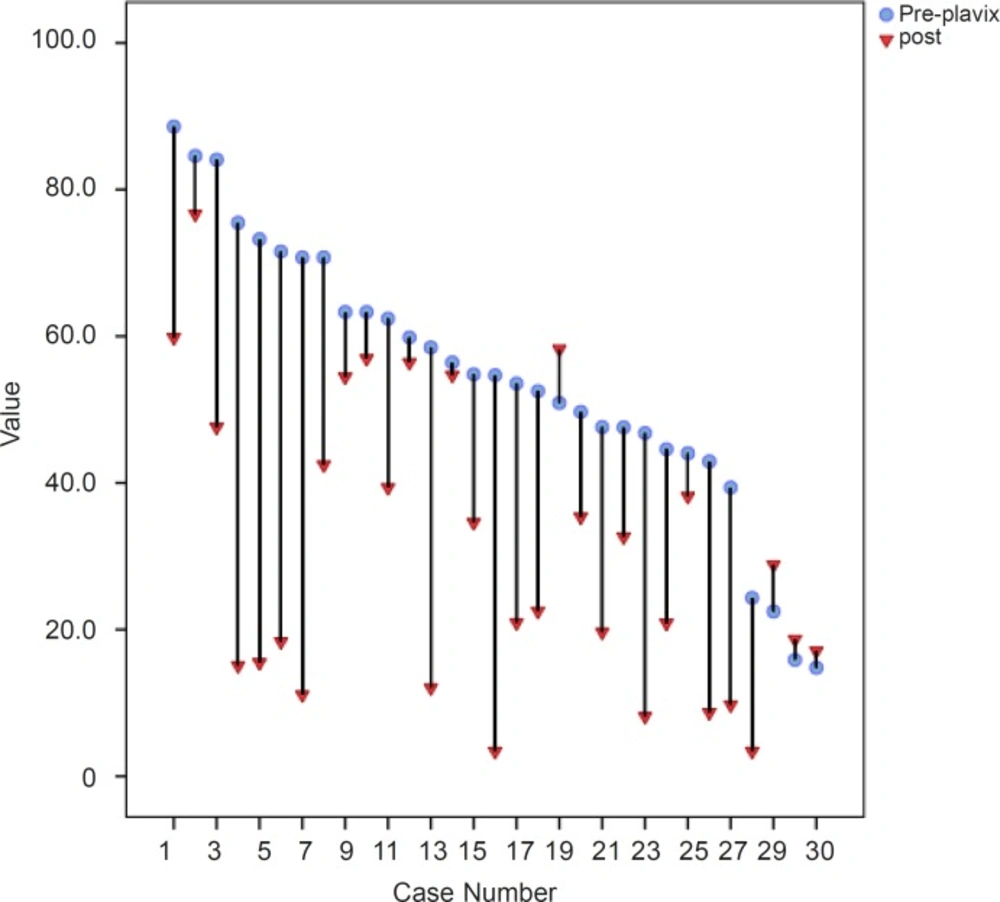
Based on our findings, 65% of patents had complete response and 22% showed semi-resistance and 13% showed complete resistance to drug. The reduction of platelet aggregation for all patients demonstrated in Figure 1.
Discussion
The percentage of drug response to clopidogrel in different populations has been reported in several studies. Lau et al. reported 22 percent resistance and 22% semi-resistance after 300 mg loading and 75 mg daily dose of clopidogrel in patients from USA which is somehow higher than our findings and might be related to lower loading dose in their study(9). They suggested the interpersonal differences in CYP3A4 activity among patients as the cause of this drug response variation (9). Lapantalo et al. used a loading dose of 600 mg and daily dose of 75 mg among Finnish patients and reported 20% resistance among 1608 patients, which is in line with our findings (10). In another study, Schulz et al. in Germany reported 20% resistance in patients undergoing angioplasty and stent placement (11). Geisler el al. reported a 5.8% resistance among German patients which is less than our findings (3).
In a study by Neubauer et al., using combination therapy with aspirin and clopidogrel among 504 patients, it was found that 30.8% of patients are resistant. Resistance to clopidogrel might be due to patient’s clinical condition or genetic factors (12). They proposed that a less active ADP receptor among some patients might cause the clopidogrel to have a somehow muted effect as ADP receptor antagonist (12). They suggested an increase of daily dose from 75 mg to 150 mg. The results of some several studies are summarized in Table 4.
| Study | N | Patients | Dose (mg, load/qd) | Prevalence% |
|---|---|---|---|---|
| Gurbel et al. (13) | 92 | PCI | 300/75 | 31-35 |
| Jaremo et al. (14) | 18 | PCI | 300/75 | 28 |
| Muller et al. (15) | 119 | PCI | 600/75 | 5-11 |
| Mobley et al. (16) | 50 | PCI | 300/75 | 30 |
| Lepantalo et al. (10) | 50 | PCI | 300/75 | 40 |
| Angiolillo et al. (17) | 48 | PCI | 300/75 | 44 |
| Matetzky et al. (18) | 60 | STEMI | 300/75 | 25 |
| Dziewierz et al. (19) | 31 | CAD | 300 | 23 |
| Lev et al. (20) | 150 | PCI | 300 | 24 |
| Angiolillo et al. (21) | 52 | Diabetics and Non-diabetics | 300 | 38 (diabetic) 8 (Non-diabetic) |
| Gurbel et al. (22) | 190 | PCI | 300 or 600/75 | 28-32 (300 mg) 8 (600 mg) |
These findings by different authors and on different populations show a variation regarding resistance to clopidogrel. Our study shows that the resistance is present among our patients but it seems that the prevalence of resistance is in line with the majority of previous studies. This study was among first studies on the resistance to clopidogrel in Iranian patients and had some limitations. The study population was limited to patients undergoing elective PCI and no emergency patient entered the study. In addition, we studied only patients who received a stent implantation during the angioplasty. Finally, our sample size of 31 patients causes our percentages regarding the prevalence of resistance to have a wide range and moderate reliability. Considering these limitations, we suggest bigger multicentric studies to cover a wider group of patients and calculate more precisely the prevalence of resistance to clopidogrel among Iranian patients.
Conclusion
Based on our findings, it seems that there is no major difference between Iranian population and other studies regarding the resistance to clopidogrel. Due to the limited number of participants in our study, further investigations with higher number of patients is recommended to more precisely calculate the percentage of resistance among the Iranian patients.