Introduction
Mycotoxins are secondary metabolites produced by microfungi that are capable of causing disease and death in humans and other animals (1). Aflatoxins (AFs) are one of the most important groups of mycotoxins which are considered to be economically and toxicologically important worldwide. AFs are produced by Aspergillus spp, mainly Aspergillus flavus, Aspergillus parasiticus, Aspergillus nomius and Aspergillus pseudotamarii (2). AFs are potent teratogens, mutagens and carcinogens, classified as Group 1 carcinogens by the International Agency for Research on Cancer, primarily affecting liver (3). However, a provisional maximum tolerated daily intake (PMTDI) of 1 ng AF/Kg body weight (bw)/ day may be used as a guidance value in the risk assessment of AF from food (4).
AFs have been detected in a number of foods including figs, nuts (peanut, walnut, almond and pistachio), cereals (wheat, maize, barley and rice), cottonseed and oil products (5). Because of potential health hazards to humans, regulatory levels have recently been documented. Currently, worldwide range of limits for aflatoxin B1 (AFB1) and total AF (AFT) are 1-20 and 0-35 ng/g, respectively (6). In Iran, the maximum tolerated level (MTL) of AFB1 for rice, wheat and peanut is 5 ng/g (6-7).
The use of reversed-phase high-performance liquid chromatography (RP-HPLC) method with fluorescence detection after immunoaffinity column (IAC) clean-up is common for AFs analysis. However, the analysis is time-consuming and total run time is approximately in the range of 6.5-15 min (8-10).
There are little data on the natural occurrence of AFs in cereals and nuts in Iran (11-12). Furthermore, exposure assessment of Iranian population to AFB1 has not been performed yet. In this study, we investigated the presence of AFB1 in various foods collected from Tehran retail market using a specifically developed and validated HPLC method. The results were then used for the first time for exposure assessment of Tehran population to AFB1.
Experimental
All reagents were of analytical grade. Solvents used for the experiments were of either HPLC or analytical grade. The standard of AFB1 was purchased from Sigma-Aldrich. The IAC for AFB1 was purchased from Vicam Company, MA, USA. The chromatographic apparatus consisted of a Wellchrom K-1001 pump, a Rheodyne Model 7125 injector and a RF10AXL fluorescence detector connected to a Eurochrom 2000 integrator, all from Knauer (Berlin, Germany). The separation was performed on Chromolith Performance (RP-18e, 100 × 4.6 mm) column from Merck (Darmstadt, Germany).
Sampling and sample preparation
Samples (rice, bread, peanut, puffed corn snack and wheat flour) were collected in June 2005 by trained personnel from various sales outlets in nine geographic zones in Tehran, Iran, according to the sampling plan for official control of mycotoxins in food (13). All samples were finely ground by mill and subsamples were stored in freezer at - 32ºC until analysis.
Preparation of AFB1 standard
Stock, intermediate and working standard solutions of AFB1 were prepared according to Stroka et al. method (14). After the preparation of standard solution of AFB1 (10 μg/mL), the concentration was determined using UV spectrophotometer. This standard was used to prepare working standards of AFB1 for HPLC analysis (14).
Aflatoxin analysis
The method used for AFB1 extraction from samples and the chromatographic conditions were based on the AOAC official method 999.07 with some minor modifications (14). Briefly, fifty grams of samples including rice, bread, wheat flour, peanut and puffed corn snack was shaken for 30 min with 5 g of sodium chloride and 300 mL of methanol:H2O (80:20 v/v). For peanut and puffed corn snack, 100 mL of n-hexane was added too. After filtration, 20 mL of the filtrate was diluted with 130 mL of deionized water and filtered through the glass microfiber filter, and 75 mL of the filtrate was used for further clean up on a Afla test IAC column. The Afla test column was preconditioned with 10 mL of phosphate buffered saline (2-3 mL/min) and then 75 mL of the diluted sample extract was passed through the column (2-3 mL/min). Finally, the column was washed with 15 mL water. For AFB1 elution from the column, a portion of 0.5 mL HPLC grade methanol was passed through the column followed by an additional portion of 0.75 mL of the same solvent one minute afterward. HPLC grade water was added to the eluent to the volume of 3 mL and 100 μL of the final solution was injected into HPLC.
For all samples, separation was performed on a monolithic column (100 × 4.6 mm) using a HPLC system equipped with a fluorescence detector. Mobile phase was water: methanol (55:45, v/v) with a flow rate of 1.2 mL/min. The fluorescence detector was operated at excitation wavelength of 365 nm and emission wavelength of 435 nm. Post-column derivatization was carried out with pyridinium hydrobromide perbromide (PBPB) at flow rate of 0.4 mL/min.
Method validation
To evaluate the reliability of the results, in addition to apply regular validation assessment to the developed method, internal quality control experiments were also performed. In each working day, a blank and a spiked sample were also analyzed. According to the recovery values, AFB1 levels were corrected for recoveries. In addition, a certified reference material (CRM) from FAPAS (UK) was analyzed.
Results and Discussion
Method validation
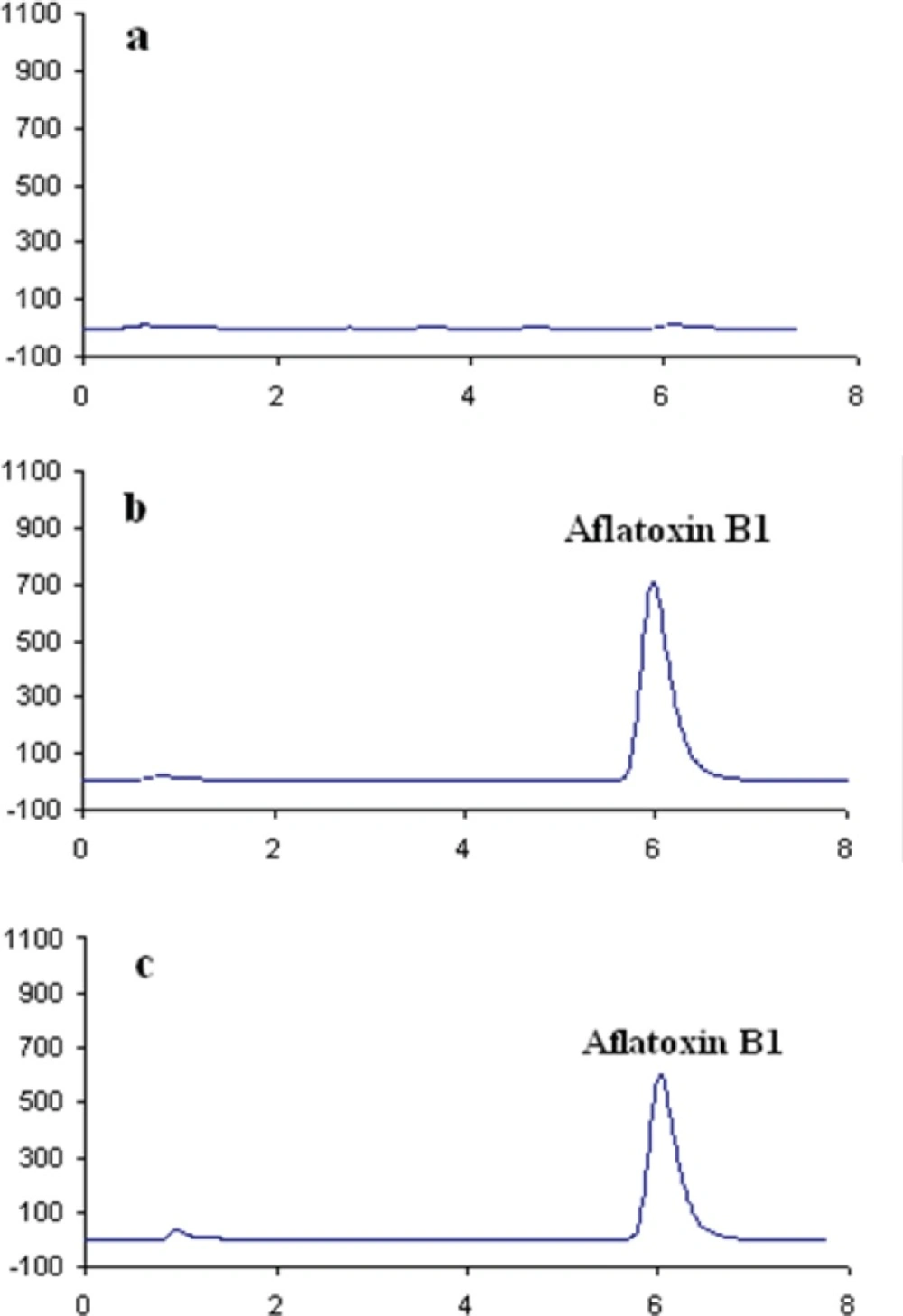
In order to perform the study on AFB1 contamination level in the five sample matrices, a specific high performance liquid chromatographic (HPLC) method was developed. Then, the method was validated in terms of linearity, limit of detection (LOD) and limit of quantification (LOQ), selectivity, precision and recovery. The method was satisfactory in terms of selectivity as an IAC was applied for purification of AFB1 which eliminated false positive results caused by interfering materials. Typical chromatograms obtained for AFB1 are shown in Figure 1.
Linearity was assessed for AFB1 over a range of 0.4-3.6 ng/g and reasonable correlation coefficients (r2 > 0.995) were obtained which indicated a good linearity of the analytical response over the specified concentration range. The estimated LOD (signal to noise ratio = 3) and LOQ (signal to noise ratio = 9) of AFB1 were 0.01 ng/g and 0.03 ng/g, respectively, which indicated that applying monolithic column improved sensitivity of the method compared to the previously reported methods (15-17).
Accuracy of the method was assessed by performing recovery experiments. Each test was performed three times and the average recoveries and relative standard deviation for repeatability (RSDr) are shown in Table 1.
| Sample | Spiking level (ng/g) | Recovery (%) | RSDr (%) |
|---|---|---|---|
| Rice | 2 | 105.4 | 9.4 |
| 5 | 97.8 | 6 | |
| 10 | 104.4 | 3.9 | |
| Mean recovery ± SD | 102.5 ± 4.13 | 4.03 | |
| Bread | 2 | 99.9 | 13.4 |
| 5 | 90.1 | 4.7 | |
| 10 | 109.8 | 13.2 | |
| Mean recovery ± SD | 99.9 ± 9.85 | 9.86 | |
| Puffed corn snack | 2 | 101.8 | 4.8 |
| 5 | 102.1 | 10.4 | |
| 10 | 100.2 | 9.6 | |
| Mean recovery ± SD | 101.4 ± 1.02 | 1.0 | |
| Peanuts | 2 | 102.6 | 17.8 |
| 5 | 98.2 | 12.6 | |
| 10 | 98.1 | 8.2 | |
| Mean recovery ± SD | 99.6 ± 2.57 | 2.58 | |
| Wheat flour | 2 | 91.2 | 7.00 |
| 5 | 94.7 | 7.4 | |
| 10 | 97.3 | 3.7 | |
| Mean recovery ± SD | 94.4 ± 3.06 | 3.24 |
Results of validation assessment of HPLC method developed for determination of AFB1 in different foods (n = 3).
The average recoveries (and RSDr) of AFB1 from spiked rice, bread, puffed corn snack, peanuts and wheat flour were 102.5% (4.03%), 99.9 (9.86%), 101.4% (1.0%), 99.6% (2.58%) and 94.4% (3.24%), respectively. These values fall well within the EU method performance criteria for AF analysis (13). The amount of AFB1 in corn CRM was 2.8 ng/g which is in the acceptable range of FAPAS Scheme (1.94-4.99 ng/g) and confirmed the accuracy of the analytical method.
| Samples | Numbers of samples in the range (ng/g) | |||
|---|---|---|---|---|
| < 0.03a | ≥ 0.03 - < 2 | ≥ 2 - < 5 | ≥ 5 | |
| Rice | 9 | 6 | 2 | 1 |
| Bread | 18 | - | - | - |
| Puffed corn snack | 7 | 11 | - | - |
| Peanuts | 4 | 10 | 2 | 2 |
| Wheat flour | 18 | - | - | - |
Natural occurrence of AFB1 in rice, bread, puffed corn snack, peanut and wheat flour samples marketed in Tehran, June 2005.
In the previous studies, the reported total run time for AFB1 was in the range of 6.5-15 min (8-10, 18). Applying monolithic column in the present work resulted in shorter analysis time (6 min) which is even faster than other published methods. Due to the application of monolithic column, less amount of organic phase was used, compared to the published methods, to optimize the resolution (19). Besides, in the present work, a mobile phase consisted of methanol:water was used, which resulted in omitting the more toxic and more expensive solvent, acetonitrile. Thus, our proposed HPLC method proved to be well suited for routine determination of AFB1 in the specified samples being around 10 samples processed per day.
Application of method to the real samples
The natural occurrence data for AFB1 in retail foods are shown in Tables 2 and 3.
| Sample | No. of samples | Positive samples (%) | Meana(± SD) | Median | Max |
|---|---|---|---|---|---|
| Rice | 18 | 9 (50) | 4.17 (9.36 ±) | 1.17 | 30.63 |
| Bread | 18 | 0 | <LOQ | - | - |
| Puffed corn snack | 18 | 11 (66.6) | 0.11 (0.11 ±) | 0.09 | 0.43 |
| Peanut | 18 | 14 (77.8) | 1.97 (2.94±) | 0.95 | 9.95 |
| Wheat flour | 18 | 0 | <LOQ | - | - |
Contamination data for AFB1 (ng/g) in rice, bread, puffed corn snack, wheat flour and peanut samples marketed in Tehran, June 2005.
| Samples | Mean a, b | Max | Daily intake | |||
| Mean | 90th percentile | 95th percentile | Max | |||
| Rice | 2.29 | 30.63 | 3.49 | 4.07 | 12.12 | 46.82 |
| Bread | NAd | NA | NA | NA | NA | NA |
| Puffed corn snack e | 0.12 | 0.43 | 0.11 | 0.19 | 0.46 | 0.43 |
| Peanuts | 1.54 | 9.95 | 0.02 | 0.05 | 0.11 | 0.14 |
| Total | - | - | 3.62 | 4.31 | 12.69 | 47.39 |
Estimated daily intake of aflatoxin B1 (ng/g) through rice, bread, puffed corn snack and peanut samples marketed in Tehran, June 2005.
Our results showed that AFB1 was present in 34 out of 90 samples (37.7%). It is also shown that none of the bread and wheat flour samples contained detectable amounts of AFB1. The maximum amount of AFB1 detected in rice, puffed corn snack and peanut were 30.63, 0.43 and 9.95 ng/g, respectively, whereas the mean positive contamination levels of AFB1 were 4.17, 0.11, and 1.97 ng/g, in rice, puffed corn snack and peanut, respectively. The level of contamination of 3 samples (one rice sample and two peanuts samples) exceeded than Iranian MTL of 5 ng/g. Khamiri et al. reported that among 156 peanut samples analyzed for AFB1, one sample (0.64%) was contaminated at the level of 491 ng/g (11). Magrine et al. determined the concentrations of AFs in 100 samples of peanut products in Brazil. There was a 50% occurrence of AFs (B1, B2, G1 and G2) in concentrations ranging from 0.5 to 113 ng/g, with 13 samples with levels above 20 ng/g (20). In our study, 11.1% of peanut samples were contaminated at the levels higher than Iranian MTL.
There is only one report on AFB1 contamination in rice in Iran (12). Mazaheri reported that AFB1 and AFT were detected in 59 of 71 rice samples. The mean of AFB1 and AFT were 1.89 and 2.09 ng/g, respectively (12). Our result is in agreement with Mazaheri study and in both studies, the mean of AFB1 were lower than Iranian MTL. In Austria, a survey has been carried out on the natural occurrence of AFB1 in rice. AFB1 was found in 24 out of 81 samples. The contamination range was between 0.45 ng/g and 9.86 ng/g. Three samples exceeded the maximum levels set in the European Union; having AFB1 concentrations of 2.16, 2.85 and 9.86 ng/g (21). In our study, just one sample exceeded the Iranian MTL.
Abdullah et al. analyzed 83 wheat flour samples in Malaysia and reported that 1.2% of samples were positive for AFB1, at a concentration of 25.6 μg/Kg (22). In our study, none of the wheat flour samples contained detectable amounts of AFB1.
Estimation of dietary intake of AFB1
This is the first study on exposure assessment of Iranian population to AFB1. Exposure to mycotoxins for each type of food depends on the mycotoxins concentration in food and the amount of food consumed. In this study, the consumption rates of rice and bread were based on a consumption survey performed in Iran since 2001-2003 (23). The average consumption of rice and bread are 107 g and 286 g per day per person in Tehran, respectively. Unfortunately, there are no data of puffed corn snack and peanut consumption in Iran so we assumed a package (65 g) per day for puffed corn snack and 1 g per day for peanut as mean daily consumption for the two products.
Estimated daily intakes of AFB1 in different foods are given in Table 4.
The tolerable daily intake for genotoxic compounds (such as AFB1) cannot be used as a safety factor, since the intake of such substances should be kept as low as reasonably achievable. However, WHO has suggested that a PMTDI of 1 ng AF/Kg bw/day may be used as a guidance value in the risk assessment of AF from food (4). Although mean estimated daily intake of AFB1 from all analyzed foods was 3.62 ng/Kg bw/day, the mean dietary intake of AFB1 from rice alone was estimated 3.49 ng/Kg bw/day which is ca 3.5 times higher than the guidance value of 1 ng AF/Kg bw/day (Table 4). Considering the high consumption rates of rice and bread in Iran, it is recommended to reduce the MTL of AFs in these foods.
The estimated probable daily intake for AFB1 in samples of peanut products analyzed in Brazil varied from 0.6 to 10.4 ng/Kg bw/day (20). In South Korea, among 88 rice samples, AFB1 was detected in 5/88 (6%) of samples with an average of 4.8 ng/g. A calculated probable daily intake of AFB1 for Koreans fell into the range of 1.19-5.79 ng/Kg bw/day (24).
Conclusion
The specifically developed HPLC method was found to be accurate, sensitive, safer and more economic, and meets EU method performance criteria for AFB1 analysis. Compared to other reported methods, it is faster and results in a remarkably low LOD of 0.01 ng/g. This method is suitable for routine analysis of AFB1. Upon applying the proposed method, in most of the samples the level of AFB1 was found to be lower than the Iranian MTL. However, due to the high consumption of foods like rice, the risk of exposure to AFB1 is higher than the guidance value of 1 ng/Kg bw/day and requires closer monitoring of the contamination in foods.