Introduction
Diabetes mellitus is an endocrinal chronic disease characterized by elevated blood glucose levels (1-3). The control of hyperglycemia is critical in the management of diabetes mellitus since in long term, acute and chronic complications can occur (1, 4, 5). One goal of therapy for diabetic patients, especially non-insulin-dependent diabetes mellitus (type 2 diabetes), is the maintenance of normal blood glucose levels after a meal (postprandial hyperglycemia) (3, 6). A therapeutic approach for decreasing postprandial hyperglycemia is to retard and reduce the digestion and absorption of ingested carbohydrates by the inhibition of carbohydrate-hydrolyzing enzymes, such as α-amylase and/or α-glucosidases, in the digestive organs (1, 5, 7, 8). Therefore, there is a need to develop compounds with enzyme inhibitory activities, for which the medicinal plants may serve as potential sources (9, 10).
The use of natural products as complementary approaches in existing medications for the treatment of diabetes mellitus is growing worldwide and many plants in different countries are known to have antidiabetic effects (11). Grover et al. reported that more than 1100 plant species have been used ethnopharmacologically or experimentally to treat diabetes mellitus (12).
Salvia is one of the largest geniuses in Labiatae family. It comprises nearly 900 species throughout the world and 58 species in Iran (13, 14). Different species of Salvia have a long history of use for medical purposes in many countries (15, 16). On the other hand, investigations conducted on various species of Salvia show that the plants have wide and diverse biological activities especially antioxidant, anti-inflammatory, spasmolytic, antidiabetic, etc (13). For example, the hypoglycemic effect of S. officinalis that has long been used in Iranian traditional medicine for the treatment of diabetes, has been supported by some scientific studies (17-21). The scientific research has confirmed the potentially α-amylase inhibitory activities of few Salvia species. Nickavar et al. showed that the extracts from S. virgata and S. verticillata had inhibitory activities on pancreatic α-amylase (22). Furthermore, based on the study of Loizzo et al., Salvia acetabulosa was a potent α-amylase inhibitor (23).
However, no investigation has been ever done on the identification of α-amylase inhibitors from Salvia genus. The aim of the present work was to identify the antidiabetic compounds and α-amylase inhibitors from Salvia virgata.
Experimental
Plant material
The aerial parts of Salvia virgata Jacq. (Synonym: Salvia sibthorpii Sibth. and Sm.) were collected from Tehran province during the flowering period in summer 2006. Voucher specimens were deposited at the Herbarium of the School of Pharmacy, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The plant was dried at ambient temperature with active ventilation.
Chemicals
All of the chemical reagents used in this study were purchased from Sigma-Aldrich Chemical Co. (France) and/or Merck Company (Germany). Acarbose was obtained from Quimica Farmaceutica Bayer, S.A. (Barcelona).
Extraction, chromatography and spectroscopy
The dried and ground plant (250 g) was extracted with ethanol 90% three times by maceration method and then, the extract was concentrated in vacuo. The crude extract (12 g) was diluted with water and partitioned with n-C6H12, CHCl3 and EtOAc, successively. The inhibitory effects of the crude extract and all fractions were studied on α-amylase activity. The ethyl acetate fraction displayed the highest inhibitory activity. The fraction was subjected to more fractionation by column chromatography on silica gel using chloroform/ethyl acetate system as the eluent. The polarity of the eluent was increased by increasing the ratio of EtOAc during the process. Fraction F10 which showed a high inhibitory activity, was purified by repeated preparative layer chromatography on coated plates with silica gel (230-400 mesh) using CHCl3/EtOAc/HCOOH (45:45:10, v/v/v) as the best developing solvent system. Finally, fraction F10 yielded a pure active compound (53 mg).
The pure compound was identified on the basis of spectral and chromatographical studies. The UV-Vis spectrum was recorded on a Shimadzu UV-Vis spectrophotometer in methanol. The NMR spectra were taken on a Varian 400 spectrometer in DMSO-d6 and chemical shifts were recorded as δ-values. The EI-MS was obtained on a Finnigan-Mat spectrometer.
α-Amylase inhibition test
The α-amylase inhibitory activity was determined using the method described previously by Nickavar et al. (22). Briefly, 1 mL of the porcine pancreatic α-amylase enzyme solution (0.5 IU/mL) in 20 mM phosphate buffer (pH 6.9) was incubated with 1 mL of each test (at various concentrations) for 30 min. The reaction was initiated by adding 1 mL of 0.5% soluble potato starch solution and the mixture was incubated for 3 min at 25°C. Then, 1 mL of the color reagent (96 mM 3,5-dinitrosalicylic acid and 5.31 M sodium potassium tartrate in 2 M sodium hydroxide) was added and the mixture was placed in a water bath at 85°C. After 15 min, the reaction mixture was diluted with distilled water and the absorbance value was determined at 540 nm. Individual blanks were prepared for correcting the background absorbance. In this case, the color reagent solution was added prior to the addition of starch solution and the mixture was then placed in the water bath immediately. Controls were representative of the 100% enzyme activity. They were conducted in an identical fashion replacing tests with 1 mL of the solvent. Acarbose, a well-known α-amylase inhibitor, was used as positive control. The inhibition percentage of α-amylase was assessed by the formulae (1):
(1)
Here, Acontrol is the absorbance of each control and Asample is the net absorbance of each sample. The net absorbance of each sample was calculated by the equation (2):
Asample =Atest- Ablank (2)
In this equation, Atest is the absorbance of each test and Ablank is the absorbance of each blank.
The Iα-amylase(%) for each sample was plotted against the logarithm of the sample concentration, and a logarithmic regression curve was established in order to calculate the IC50 valve.
Results and Discussion
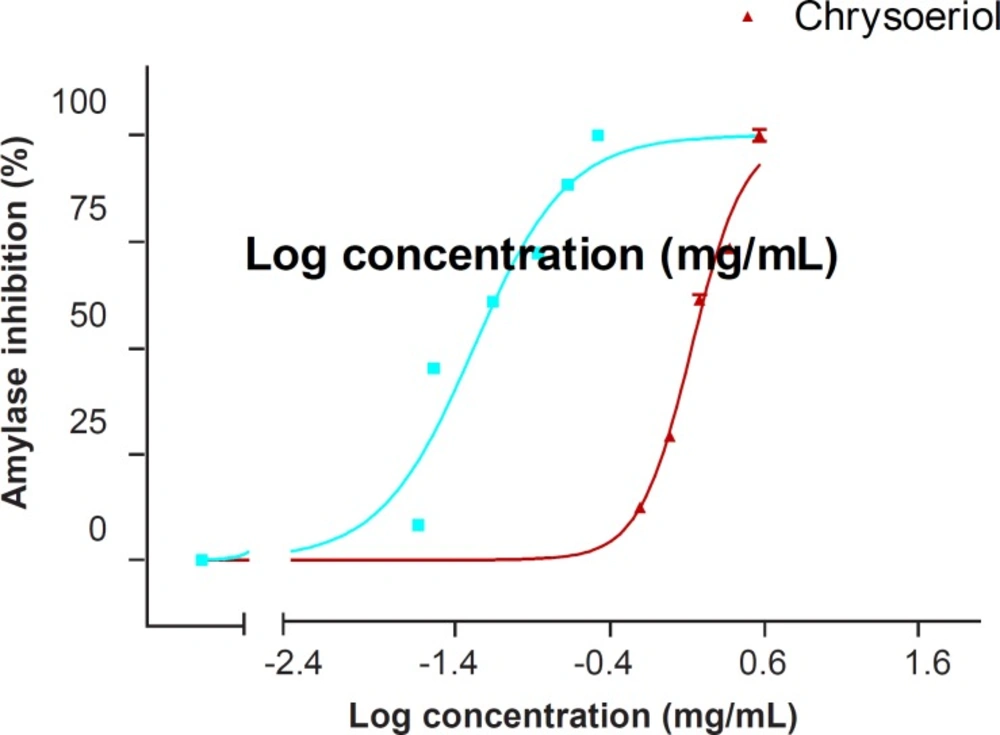
The ethanol extract of S. virgata showed a dose-dependent inhibitory effect on the α-amylase activity [IC50 = 19.08 (18.61-19.56) mg/mL] (Table 1).
| Concentration | Inhibition (%)a | IC50b |
|---|---|---|
| Extract (mg/mL) | ||
| 36.00 | 83.69 ± 1.15 | |
| 28.80 | 74.28 ± 0.49 | |
| 23.04 | 70.92 ± 0.74 | 19.08 (18.61-19.56) mg/mL |
| 18.43 | 30.83 ± 1.18 | |
| 14.75 | 18.37 ± 0.61 | |
| Chrysoeriol (mM) | ||
| 3.48 | 96.28 ± 1.38 | |
| 2.23 | 70.62 ± 0.95 | |
| 1.42 | 58.98 ± 1.20 | 1.27 (1.21-1.33) mM |
| 0.91 | 28.05 ± 0.80 | |
| 0.58 | 11.91 ± 0.85 | |
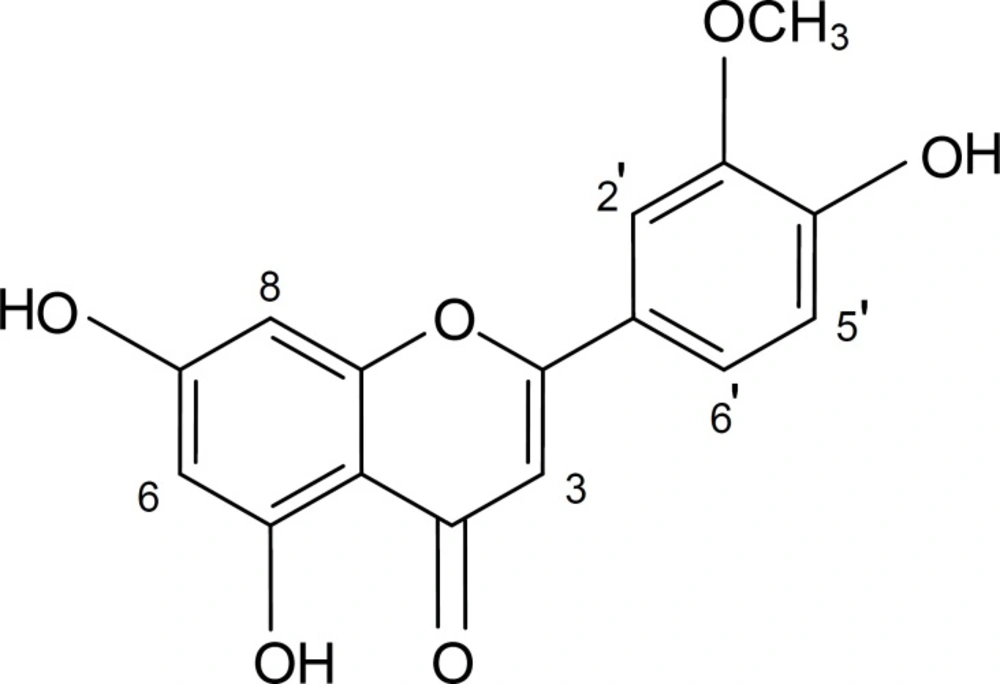
α-Amylase inhibitory activities and IC50 values of the aerial parts of S. virgata and its active compound chrysoeriol.
In order to identify the active components, solvent-solvent partition performed with n-C6H12, CHCl3 and EtOAc, successively. The ethyl acetate fraction revealed the highest activity so it was selected for further separation. The chromatographical analysis of the ethyl acetate fraction showed flavonoid compounds. The most active flavonoid compound was isolated as the pale yellow amorphous powder (53 mg). It had ca R f = 0.7 on TLC (silica gel 60) with CHCl3/ EtOAc/HCOOH (45:45:10, v/v/v). The spectroscopic data for the compound were as follows:
UV-Vis: λmax (in CH3OH) = 266 (sh.), 354 nm
1H-NMR (400 MHz, in DMSO-d6), δ: 3.93 (3H, s, OCH3-3’), 6.18 (1H, br. s, H-6), 6.45 (1H, br. s, H-8), 6.58 (1H, s, H-3), 6.89 (1H, d, J = 8 Hz, H-5’), 7.37 (1H, br. s, H-2’), 7.40 (1H, d, J = 8 Hz, H-6’).
13C-NMR (100 MHz, in DMSO-d6), δ: 54.8 (OCH3), 93.2 (C-8), 98.1 (C-6), 102.1 (C-3), 102.9 (C-10), 110.5 (C-2’), 115.7 (C-5’), 120.6 (C-6’), 130.4 (C-1’), 146.1 (C-3’), 148.8 (C- 4’), 154.4 (C-2), 155.7 (C-9), 161.3 (C-5), 167.6 (C-7), 171.0 (C-4).
EI-MS (70 eV), m/z (I %): 300 (10%), 286 (82%), 153 (26%), 151 (20%).
The spectral data of the compound showed that it was chrysoeriol (Figure 1) and all of its data were matched with those reported in the literature (24, 25) .
In this study, chrysoeriol inhibited α-amylase activity in a dose-dependent manner. The IC50 values for α-amylase inhibition by chrysoeriol and acarbose (as the positive control) were 1.27 (1.21-1.33) mM and 0.049 (0.042-0.056) mM, respectively (Figure 2 and Table 1).
The genus Salvia generally produces a variety of phenolic metabolites, especially flavonoids, which have received much attention due to their relevant biological properties (13). Phytochemical literature survey on S. virgata shows the occurrence of few hydroxycinnamic acid derivatives (such as rosmarinic acid, caffeic acid, etc.) and flavonoids (such as salvigenin, luteolin and its glycosides, luteolin 7,3’,4’-trimethyl ether, etc.) (13, 26, 27). On the other hand, chrysoeriol has already been isolated from few Salvia species including S. candidissima, S. dorrii, S. lavandulaefolia, S. mirzayana, and S. palaestina (13). However, to the best of our knowledge, this is the first report on the isolation and identification of chrysoeriol from S. virgata and the inhibitory effect of the compound on α-amylase activity.