Introduction
Crocus sativus L. (saffron), a culinary spice, is utilized for its flavouring, colouring, aromatic and medicinal effects (1-3). Saffron quality depends on the presence of its three major constituent in its stigmas. Picrocrocin (4-(β-D-glucopyranosyloxy)-2, 6, 6-trimethyl- 1-cyclohexene-1-carboxaldehyde, C16H26O7), a monoterpene glycoside, is the main bitter principle of saffron. Safranal (2, 6, 6-trimethyl-1, 3-cyclohexadiene-1-carboxaldehyde), a monoterpene aldehyde, is a main constituent of the essential volatile oil responsible for saffron odor and aroma. Picrocrocin releases the aglycone, by β-Glucosidase action which is changed to safranal through the drying procedure and also is formed during the storage of saffron (2, 4-6). Crocin (glucosyl ester of crocetin) is responsible for colouring properties of saffron (2, 7-9).
Safranal has shown different pharmacological activities such as bronchodilation (10, 11), reduction of ischemia-reperfusion injury in kidney (12), brain (13, 14 ) or skeletal muscle (15), prevention of gentamicin- or hexachlorobutadiene-induced nephrotoxicity (16, 17), reduction of subacute toxicity of diazinon (18), hypnotic and antianxiety (19), hypotensive (20), anticonvulsant (21-23), antidepressant (24), genoprotective (25), antioxidant (26, 27), antinociceptive (28), antitussive (29) and anticancer activities (30).
The LD50 values of saffron stigma and petal extracts by intraperitoneal administration in mice were 1.6 and 6 g/kg, respectively. In a subacute toxicity study, both extracts decreased the value of hematocrit, hemoglobin and erythrocytes, however, the stigma extract did not cause any significant pathological effects in different organs (30). In humans, saffron with doses between 1.2 and 2 g induced nausea, vomiting, diarrhea and bleeding (32). High oral and intraperitoneal doses of crocin (3 g/kg) did not cause death within 2 days of study in mice (33). At high doses (200 and 400 mg/day), saffron tablets changed some hematological and biochemical parameters in healthy adult volunteers. However, these changes were in normal ranges and they were not clinically important (34). Despite the wide use of saffron in many countries as an herbal remedy and the useful pharmacological effects of safranal, few studies have been published in the literatures about their toxicological profiles. Therefore, the aims of the present study were to assess the acute and subacute oral and intraperitoneal toxicity of safranal in rodents.
Experimental
Chemicals
Safranal was purchased from Fluka Chemie AG (Buchs, Switzerland). Enzymatic reagent kits for determination of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactic acid dehydrogenase (LDH) and creatine phosphokinase (CPK) were purchased from Greiner Bio-One and alkaline phosphatase, bilirubin, blood urea nitrogen (BUN), albumin, cholesterol and triglyceride were purchased from Pars Azmoon Co. All other chemicals and solvents used throughout this study were of analytical grade.
Animals
Male Wistar rats (weighing approximately 200–250 g) and BALB/c mice of both sexes (weighing approximately 25–30 g) were obtained from the animal house of the Pharmaceutical Sciences Research Center of Mashhad University of Medical Sciences, Mashhad, Iran. Animals were housed in a colony room under a 12/12 h light/dark cycle at 21 ± 2°C and had free access to water and food. All animal experiments were approved by the Animal Care Committee of Mashhad University of Medical Sciences.
Acute toxicity of safranal in mice and rats
Animals randomly were divided into several groups (n = 6/sex). The first group (control group) received saline and other groups were treated with different doses of safranal by oral or intraperitoneal route. Following administration, animals were observed for signs of toxicity and mortality for a period of 48 h after treatment. The lethal dose (LD50) was estimated according to the method described by Litchfield and Wilcoxon method (PHARM/PCS software version 4).
Subacute toxicity
Twenty four male rats were randomly divided into four groups (n = 6). Animals were given saline as a control. According to LD50 value, animals received safranal at the doses of (0.1, 0.25 or 0.5 mL/kg/day, orally) for 21 days. The weight of the body was determined weekly. Animals were observed for general behavioral and signs of abnormalities during the experiment duration.
Blood sampling
After 21 days, animals were anaesthetized by chloroform. Blood samples were collected by cardiac puncture into sterile tubes with anticoagulant (EDTA) for hematological tests and without anticoagulant tubes for biochemical tests. Blood samples (without anticoagulant) tubes were centrifuged at 5000 rpm for 15 min and serum was separated.
Hematological and biochemical analyses
For hematological analysis, white blood cell (WBC), red blood cell (RBC), hemoglobin concentration, hematocrit, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC) and platelets were measured using an automatic hematocyte analyzer.
The serum was analyzed for alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactic acid dehydrogenase (LDH), creatine phosphokinase (CPK), total bilirubin, serum glucose, total cholesterol, triglyceride, albumin, serum urea nitrogen (BUN), creatinine using commercial colorimetric kits.
Histopathological analysis
After blood collection for hematological and biochemical tests, organ tissues like heart, liver, kidneys, spleen and lungs were carefully removed for histological tests. After macroscopic study, these organs were fixed in a 10% buffered formalin solution. The prepared sections were subjected to hematoxylin–eosin for histopathological observations under an optical microscope.
Statistical analysis
Data were determined as mean ± SEM. All data were analyzed using analysis of variance (ANOVA) followed by Tukey- kramer. Statisticsl significance was defined as the p-values less than 0.05 (p < 0.05).
Results
Acute toxicity studies
The intraperitoneal LD50 values of safranal in male and female mice were 1.48 mL/kg (0.96- 2.29) and 1.88 mL/kg (1.03- 3.44), respectively and the maximum non-fatal dose for both sexes was 0.75 mL/kg. The oral LD50 values of safranal in male and female mice were 21.42 mL/kg (7.4- 64.29) and 11.42 mL/kg (7.44- 17.53), respectively and the maximum non-fatal dose for female mice was 5 mL/kg. The intraperitoneal and orally LD50 values of safranal in male Wistar rats were 1.5 mL/kg (1.06- 2.13) and 5.53 mL/kg (3.68- 8.3), and the maximum non-fatal doses were 0.75 mL/kg and 3.5 mL/kg, respectively.
The adverse effects and lethality increased progressively with increasing doses by both routes. All of the mice and rats died after receiving doses of 5 and 7 mL/kg intraperitoneally and deaths occurred within 2 days. In mice and rats, safranal interaperitoneally did not cause any lethality at doses of 0.5 and 0.75 mL/kg.
Oral subacute toxicity
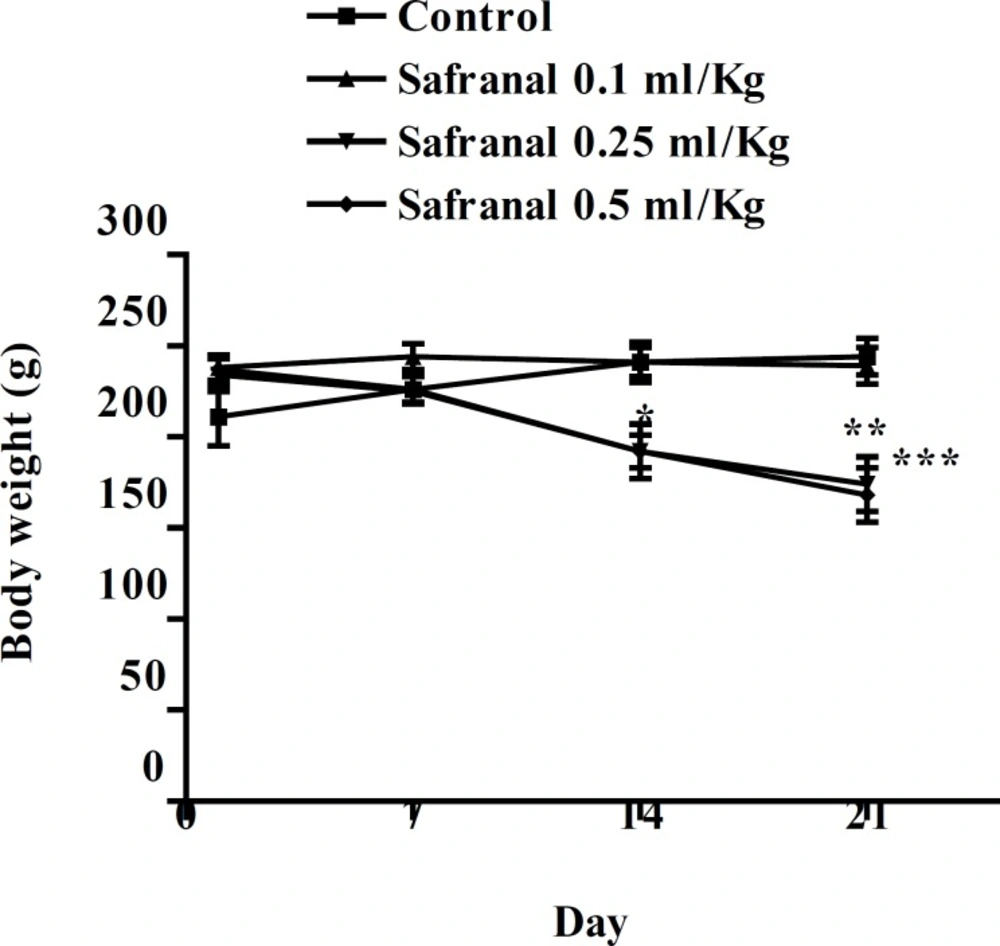
After approximately 5-7 min of treatment, animals became excited and exhibited hyperactivity; this was followed by sedative effects, relaxation and decrease in locomotor activity. Also asthenia, anorexia, decrease in food and water consumption and weight loss were observed after the oral treatment, and were more significant at higher doses (figure 1). At the end of the experiment, the body weights of animals were significantly decreased.
As shown in Table 1, RBC, hemoglobin, hematocrit, and platelets were significantly decreased in all doses of treatment groups compared to the control group, however the hematological analysis showed no significant difference of MCV, MCH, MCHC and WBC in treated groups in relation to the control.
| Parameter | Control | Safranal | Safranal | Safranal |
|---|---|---|---|---|
| WBC (×103μL) | 6.13 ± 0.82 | 6.1 ± 0.37 | 7.6 ± 0.57 | 5.75 ± 0.94 |
| RBC (×106/μL) | 8.23 ± 0.17 | 7.5 ± 0.21* | 4.94 ± 0.19 *** | 3.7 ± 0.2 *** |
| Hemoglobin (g /dL) | 15.28 ± 0.23 | 13.18 ± 0.33 | 8.56 ± 0.33 | 6.05 ± 0.46 *** |
| Hematocrit (%) | 44.7 ± 1.02 | 37.86 ± 0.94 *** | 26.58 ± 0.72 *** | 22.66 ± 0.71 *** |
| MCV (fL) | 54.03 ± 0.53 | 50.5 ± 0.3 | 51.25 ± 1.51 | 51.85 ± 1.11 |
| MCH (pg) | 18.63 ± 0.22 | 17.61 ± 0.13 | 17.91 ± 0.37 | 18.06 ± 0.21 |
| MCHC (g/dL) | 34.53 ± 0.26 | 34.85 ± 0.11 | 34.06 ± 0.46 | 35.26 ± 0.3 |
| Platelets (×103 /μL) | 910 ± 41.36 | 689.33 ± 76.3 * | 269.16 ±38.78*** | 84.66 ± 11.56*** |
The subacute oral administration of safranal (daily for 21 days) did not show any significant difference in some biochemical profiles such as serum glucose, total bilirubin, serum creatinine, albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), creatine phosphokinase (CPK) and total bilirubin (Tables 2). However, the levels of total cholesterol, triglyceride showed a significant reduction in all doses and alkalin phosphatase (ALP) was decreased in animal given 0.5 and 0.25 mL/kg of safranal. Safranal induced an increase in the level of lactic acid dehydrogenase (LDH) and serum urea nitrogen (BUN) in the treated group at high dose (0.5 mL/Kg) (Tables 2).
| Parameter | Control | Safranal | Safranal | Safranal |
|---|---|---|---|---|
| Glucose (mg/dL) | 145.5 ± 10.93 | 127.83 ± 37.41 | 152 ± 10.35 | 154.16 ± 20.71 |
| BUN (mg/dL) | 35.66 ± 1.3 | 36.66 ± 0.66 | 37.5 ± 2.89 | 73.33 ± 14.22 ** |
| Creatinine (mg/dL) | 0.43 ± 0.02 | 0.45 ± 0.02 | 0.38 ± 0.03 | 0.36 ± 0.2 |
| Cholesterol (mg/dL) | 59.33 ± 2.48 | 62 ± 2.43 | 46.33 ± 2.1 ** | 37.5 ± 1.45 *** |
| Triglycerides (mg/dL) | 74.33 ± 8.89 | 45 ± 4.34 ** | 43.66 ± 4.23 ** | 40.33 ± 4.16 ** |
| LDH (IU/L) | 248.16 ± 59.05 | 210.83 ± 27.4 | 211.5 ± 28.78 | 434.66 ± 50.77 * |
| CPK (IU/L) | 371 ± 84.8 | 290.5 ± 26.55 | 533.2 ± 55.13 | 554.6 ± 114.59 |
| ALT (IU/L) | 69.16 ± 6.41 | 70 ± 6.41 | 58.16 ± 3.04 | 169.33 ± 134.16 |
| AST (IU/L) | 87.66 ± 9 | 99.33 ± 7.94 | 117.5 ± 14.24 | 123.4 ± 40.15 |
| ALP (IU/L) | 65.75 ± 9.28 | 63.66 ± 3.52 | 35.2 ± 2.69 ** | 21 ± 3.96 *** |
| Albumin (g/dL) | 3.55±0.07 | 3.5±0.05 | 3.31±0.09 | 3.51 ± 0.14 |
| Total bilirubin (mg/dL) | 0.45 ± 0.02 | 0.38 ± 0.01 | 0.38 ± 0.03 | 0.36 ± 0.02 |
Pathological examinations of the tissues indicated that there were no detectable abnormalities and alterations in the microscopic examination of the organs such as heart, liver and spleen in comparison to the control. However, histological examinations studies indicated abnormalities and toxic effects in kidneys and lung, especially at a higher dose (0.5 mL/Kg). Edema and cytolysis were noticed in the kidney and progressed emphysema and lymphocyte infiltration were observed in lungs.
Discussion
Saffron and its constituents are widely used as spice and medicinal plant in folk medicine. Medicinal herbs usually have different constituents that possess the potential to cause useful and/or harmful effects. To evaluate the appropriate effect of safranal and for its further development as a therapeutic medicine, we needed to assess both acute and subacute toxicity for this compound. The results of the acute toxicity study indicated that LD50 values of safranal varied according to species and route of administration. According to the toxicity classification, substances with a LD50 value within the range of 1–5 g/kg are considered a practically low-toxic and substances that show LD50 higher than 5.0 g/kg may be considered practically non-toxic (35, 36). The calculated safranal LD50 values of intraperitoneal route in male and female mice and male Wistare rats were in the range of 1–5 g/kg. Thus, safranal should be considered as practically low-toxic in acute intraperitoneal route. In regard to LD50 values in mice and rats by the oral route (> 5.0 g/kg), the present investigation shows that safranal is practically non-toxic in acute oral administration. There were no sex-related differences in the toxicity of safranal (p > 0.05). Due to variations in systems of detoxification and differences in absorption, distribution, excretion mechanisms in different animal species, there are species differences in response to toxic substances (37). Safranal was more toxic when administrated intraperitoneal as compared to the oral route. The reason for the high toxicity of safranal by the intraperitoneal route compared to the oral administration, might be due to more first pass effect and lower rate of absorption with the oral treatment.
In the subacute toxicity study, safranal at doses of 0.1, 0.25 and 0.5 mL/kg by oral rout for 21 days did not cause any death in rats. However, treated animals showed some evidence of toxicity. Behavioral changes such as hyperactivity and excitation after 5-7 min of treatment might be related to the irritant nature of most essential oils.
Safranal showed sedative effects and reduction of locomotor activity. Safranal has an interaction with GABA A receptors (22) and it was demonstrated that safranal has hypnotic and anti anxiety effects (19).
The subacute tests indicated a significant decrease in body weight. It may be due to safranal effect on reduction of appetite which leads to a reduced food consumption. The daily supplementation over 8 weeks with a food supplement of a patented extract of saffron stigma significantly reduced the frequency of snacking events and a slight body weight loss in healthy women was observed (38).
In this study, the results of the hematological parameters showed a significant decrease of RBC, hemoglobin, hematocrit, and platelets in the treated groups as compared with the control group. Our results are quite similar to results of other investigating groups that indicated the reduction of hemoglobin and hematocrit levels and total RBC count by saffron (31, 39).
In addition, the treatment with safranal did not alter the biochemical parameters, except for an increase in lactic acid dehydrogenase (LDH) and serum urea nitrogen (BUN) levels in treated groups and decrease in total cholesterol, triglyceride and alkaline phosphatase (ALP). Pathological evaluations of the tissues did not indicate changes and abnormalities in organs except in kidney and lungs.
In this study, there were no changes on the common markers of liver toxicity such as levels of the liver enzymes ALT and AST and bilirubin. Thus, it may be presumed that safranal did not cause significant damage to this organ. The reduction of alkaline phosphatase level might be related to malnutrition and decrease in food and water consumptions and might not be attributed to hepatotoxicity.
Safranal treated rats showed an increase on blood urea nitrogen (BUN). The levels of BUN and creatinine are good indicators of renal function. The increased level of BUN in the treated groups can be attributed to renal damage and this is further confirmed by the pathologic findings of this organ. However, in this study no difference in levels of serum creatinine was observed.
The levels of blood cholesterol and triglyceride significantly reduced in the tested groups, as compared to the control group, indicating that safranal has hypolipidemic effects. In addition, the reduction of blood cholesterol and triglyceride might be related to decrease in body weight, diminished food and malnutrition.
The data showed no change in the level of creatine phosphokinase (CPK) in control and all administrated groups. The creatine phosphokinase (CPK) is one of the indicators available for the diagnosis of cellular damage to the heart (40, 41). No significant changes in creatine phosphokinase (CPK) values and no histopathologic damage in the heart indicates that the sub-acute treatment of safranal at the above mentioned doses did not induce any damage to the heart. The level of lactic acid dehydrogenase (LDH) was increased in the treated group at the high dose (0.5 mL/Kg). Since many tissues contain LDH, elevated total LDH is considered a nonspecific indication of cellular damage.
The results of the previous study on acute and subacute toxicity of crocin indicated that crocin at pharmacological doses did not demonstrate any mortality and damages to all the major organs of the body (33) but in this study safranal showed mortality in mice and rats as well as abnormalities in kidney and lungs and also safranal induced decrease in hematological paremeters such as RBC and hemoglobin. With regards to these data, safranal showed more toxicity than crocin.
In conclusion, this study presented the results on the acute and subacute toxicity of safranal, a constituent of Crocus sativus that can be very useful for any future in-vivo and clinical studies. According to the LD50 data, safranal was low-toxic in acute intraperitoneal route and practically non-toxic in acute oral administration in both mice and rats. The data suggest that the oral administration of safranal, in subacute toxicity test did not induce any toxic effects in many organs except for kidneys and lungs. However, further long-term and also other routs of administration studies are necessary to re-evaluate these results.