Introduction
Ulcerative colitis (UC) is characterized by chronic and relapsing inflammation of the intestines, resulting in diarrhea, abdominal pain, and a variety of other symptoms (1), and impacts negatively on the quality of life. Thus, development of a more effective therapy to treat UC disease is necessary. Huangqing-Tang decoction, described by Zhang Zhongjing (150 to 219 A.D., in Chinese Eastern Han Dynasty), has been used for the treatment of UC disease for thousands of years. Radix Scutellariae and Radix Paeoniae alba are the key ingredient herbs present in this decoction, The combination of Radix Scutellariae and Radix Paeoniae alba (3:2 g/g) renders its features, such as the heat-clearing, antiinflammatory, antidiarrheic and antinociceptive effects. Apparently, it is essential to study the synergistic interaction of Radix Scutellariae and Radix Paeoniae alba for elucidating the substantial foundation of Huangqing-Tang.
Radix Scutellariae has been widely used in Traditional Chinese Medicine (TCM) for the treatment of inflammation, fever, hepatitis, allergic diseases and hypertension, and is comprised of the flavonoids, baicalin (BG), wogonoside (WG), baicalein (B) and wogonin (W) as the active ingredients (Figure 1) (2–4).
To date, the pharmacokinetic (PK) profiles of BG and WG in plasma, brain and eyes of rats and rabbits have been reported (5–11). It is generally assumed that baicalin is poorly absorbed from the gastrointestinal tract in its native form and must be hydrolyzed by microflora enzymes (bacterial-glucuronidase) in gut to its aglycone - baicalein, then reconverted back to baicalin. Similar to baicalin, wogonoside is also firstly metabolized by microflora enzymes and reconverted, and is subsequently excreted mainly in conjugated form following administration of Radix scutellariae extract in rat and human. Baicalin and wogonoside demonstrated bimodal phenomenon in the plasma profile (12, 13). After oral administration of baicalein, levels of baicalein were negligible, whereas the glucuronides/sulfates conjugates of baicalein were predominant in the plasma (14, 15). Various case reports on herb–drug interactions or herb–herb interactions in-vivo have been published in recent years, in addition to some reports on the influence of disease condition on the pharmacokinetic characteristics of drug (16–19). Obviously, drug–herb or herb–herb interactions are common phenomena in TCM. A better understanding of the pharmacokinetics of herbal interactions and herbs in diseased rats would be helpful in formulating rational dosage regimens (20).
However, to the best of our knowledge, there are no published studies reporting the pharmacokinetics of BG, WG, B, and W after oral administration of pure baicalin, Radix Scutellariae and Scutellariae-Paeoniae couple extracts to normal rats, let alone to UC rats. The syndrome of the trinitro-benzene-sulfonic acid)TNBS(-induced colonitis model is similar to the spontaneous UC. This animal model has been used extensively in studies of colonitis and its complications, including the altered drug pharmacokinetics under the colonitis conditions (21–26). The aim of this study is to explore the pharmacokinetic differences of the four flavonoids after oral administration of pure baicalin, Radix Scutellariae extract and Scutellariae-Paeoniae couple extract to rats and to compare the pharmacokinetic parameters of UC rats with those of normal rats.
Experimental
Materials and chemicals
Radix Scutellariae, Radix Paeoniae alba were purchased from Bozhou Medicine Company (Anhui, China) and authenticated by Prof. Feng Li from the College of Pharmacy, University of Traditional Chinese Medicine (Liaoning, China). The voucher specimens (No.20081205, 20081211) have been deposited in Liaoning University of TCM. Pure baicalin was obtained from Prof. ZhenQiu Zhang (Dept. of Chemistry in our institute, Liaoning University of TCM). Baicalin and wogonin were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The internal standard (IS) arctigenin was supplied by Prof. DeQiang Dou (Department of Phytochemistry in our institute). Baicalein was purchased from Tianjin Yifang Science )Technology Co. Ltd. (Tianjin, China). Wogonoside was purchased from Shanghai Ronghe Science) Technology Co. Ltd. (Shanghai, China). The solvents used for chromatographic analysis were HPLC grade and were purchased from Fisher Company Inc., USA. Deionized water was prepared in a Mill-Q academic water purification system (Millipore, Bedford, MA, USA). All the other reagents were of analytical grade and provided by Kermel Chemical Co. (Tianjin, China).
Apparatus and chromatographic conditions
The concentrations of the four flavonoids in plasma were assayed using reverse-phase high performance liquid chromatography (Agilent 1100 series) equipped with a variable wave length UV detector and pump (Agilent model G1314A VWD). Separation was accomplished on a Eclipsel XDB-C18 column (250 mm × 4.6 mm, 5 μm particle size). The mobile phase was composed of acetonitrile (A) : 0.1% phosphoric acid water (B) (0 → 10 min, 16 : 84; 10 → 15 min, 22 : 78; 15 → 25 min, 25 : 75; 25 → 30 min, 34 : 64; 30 → 50 min, 42 : 58, v/v) at a flow rate of 1.0 mL/min with gradient elution. The column temperature was 30 °C. The detector was set at 278 nm.
Preparation of radix scutellariae and scutellariae-paeoniae couple extracts
Radix Scutellariae and Radix Paeoniae alba were mixed in the ratio of 1.5 : 1 g/g and the total weight was 200 g. The mixture was decocted twice by refluxing with 70% ethanol (1 : 10 and then 1 : 8 w/v) for 1 h, and the solution obtained was concentrated to give an extract of 60.5 g. Radix Scutellariae (200 g) was treated as above to provide an extract of 43.3 g. The dried powder was stored at 4 °C before use. For estimation of the administered dose, the concentrations of baicalin, wogonoside, baicalein and wogonin in the extracts and pure baicalin were quantitatively determined. The extract powder (0.10 g) was ultrasound with 100 mL 60% methanol for 30 min. The solution was filtered through 0.45 μm organic membrane before HPLC analysis. The contents of BG, B, WG and W were determined to be 17.3, 7.6, 2.4 and 1.7 % in Scutellariae-Paeoniae couple extract and 43.6, 19.1, 6.2 and 4.5% in Radix Scutellariae extract, respectively. The content of baicalin was 95.5 % as pure baicalin.
Animals
Male Sprague–Dawley rats, weighing 250-280 g, were obtained from the Experimental Animal Department of Dalian University (Dalian, China). Animal welfare and experimental procedures were strictly in accordance with the Guide for the Care and Use of Laboratory Animals (US National Research Council, 1996) and the related ethics regulations of Liaoning University of TCM. Rats were housed in an air-conditioned animal quarter at a temperature of 22 ± 2 °C and a relative humidity of 50 ± 2%. All animals received food and water ad libitum. The animals were acclimatized to the facilities for five days, and then fasted with free access to water for 24 h prior to each experiment.
Animal model
Colitis was induced in rats according to the model and method described by Morris et al. (27). Briefly, rats were lightly anesthetized with 10% chloral hydrate solution, and then a medical-grade polyurethane cannula for enteral feeding (external diameter 2 mm) was inserted into the anus and the tip was advanced to 8 cm proximal to the anal verge. TNBS (were obtained from Shanghai Jinmai Co. Ltd. Shanghai, China.) dissolved in 100% ethanol was instilled into the colon through the cannula (at a dose 80 mg/kg). Following the instillation of the hapten, the rats were maintained in a head-down position for an additional 30 sec to prevent leakage of the intra colonic instillation. Then the rats were returned to their cages. The rats were checked daily for behavior, body weight, and stool consistency. Successful replication of the model of UC was characterized by reduction in activities, apathy and occult blood in stool. These were the criteria used for evaluating UC rats.
Biosample collection
The UC rats were divided into three subgroups randomly, the pure baicalin, Radix Scutellariae extract and Scutellariae-Paeoniae couple extract subgroup. The normal rats were divided in the same way. Each group was respectively administrated an oral dose of 200 mg/kg baicalin (in the form of pure compound or co-administrated mixture, 0.21 g/kg pure baicalin, 0.46 g/kg Radix Scutellariae extract and 1.16 g/kg Scutellariae-Paeoniae couple extract), All of the compounds were suspended in water and homogenized using ultrasonic technology just prior to dosing. Blood samples (0.5 mL) were collected into a heparinized tube via the oculi chorioideae vein before drug administration and at 0.083, 0.25, 0.5, 0.75, 1.0, 2.0, 4.0, 8.0, 12.0 and 24.0 h after drug administration, and were centrifuged at 4000 rpm for 10 min for the separation of plasma from the red blood cells. The supernatant was decanted carefully and was stored at − 40 °C until analysis.
Biosample preparation
An aliquot of 15 μL arctigenin (32.0 μM in methanol) and 10 μL HCl solution (0.1 mM) were added into 100 μL plasma, and then spiked with methanol 75 μL and acetonitrile 100 μL by vortex mixing for 5 min. The mixture was centrifuged at 10000 rpm for 15 min. An aliquot of 50 μL of the supernatant was injected into the HPLC system.
Assay validation.
Known amounts of baicalin, baicalein wogonoside, wogonin and IS were added into 100 μL of blank plasma to prepare the following series of standards. The calibration curves showed good linearity in the range of 0.123–31.6 μM for baicalin, 0.288–73.6 μM for wogonoside, 0.133–8.50 μM for baicalein, 0.062–7.9 μM for wogonin. The extraction recoveries and matrix effects at three quality control (QC) concentrations were assayed in sets of six replicates. The CVs of the recoveries were less than 20% at low concentration, and less than 15% at medium and high concentrations. The recoveries were all more than 70%. The recovery for IS was 98.1 ± 3.50%. Accuracy and precision of the method were investigated by intra-day and inter-day data via comparing the mean of five replicates of each QC sample. Both CV for precision evaluation and RE for accuracy evaluation were less than ±18%. The stabilities of baicalin, wogonoside, baicalein and wogonin were determined by analyzing QC samples at three concentrations exposed to encounter during sample storage. The corresponding relative errors were less than ± 6% for samples at three concentrations for each reference standard, respectively. Results of the stability test showed that under the experimental conditions the samples were stable throughout the testing process.
Data analysis
The plasma concentration vs. time data of baicalin and wogonoside were analyzed by non-compartmental model using the nonlinear least squares regression program WinNonlin (Scientific Consulting Inc., Cary, NC, U.S.A.). All the results were expressed as means ± standard deviation (S.D.). The Microsoft Excel Program Drug and Statistics version 2.0 software (T.C.M. Shanghai, China) were used to calculate the pharmacokinetic parameters, such as the area under curve (AUC), the maximum plasma concentration (Cmax) and the corresponding time (Tmax), the half-life of absorption (T1/2), etc. Statistical analysis of pharmacokinetic parameter estimates was performed using a two-tailed nonparametric t-test. Differences between means were considered significantly if p ≤ 0.05.
Results and Discussion
HPLC assay
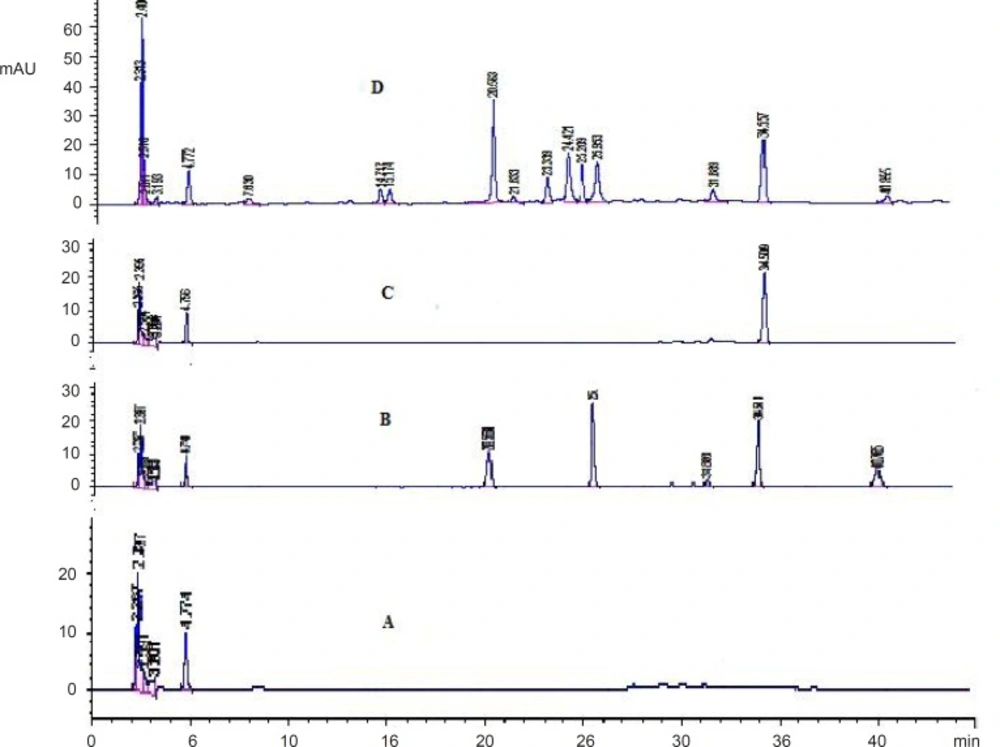
The selectivity of the method was evaluated by analyzing blank plasma samples prior to administration. The chromatograms were free of interfering peaks at the retention times of IS (34.5 min) and baicalin (20.6 min), wogonoside (25.9 min), baicalein (31.9 min) and wogonin (40.8 min). Figure 2 showed the representative chromatograms of blank plasma sample (A), blank plasma sample spiked with baicalin, wogonoside, baicalein, wogonin and IS (B), blank plasma sample spiked with IS (C), plasma sample (1.5 h) after oral administration of Scutellariae-Paeoniae couple extract (D). Under the established chromatographic condition, no interfering peaks were observed at the elution times of baicalin, wogonoside, baicalein, wogonin and the internal standard.
Pharmacokinetics of baicalin and wogonoside after oral administration of pure baicalin, Radix Scutellariae and Scutellariae-Paeoniae couple extracts to rats.
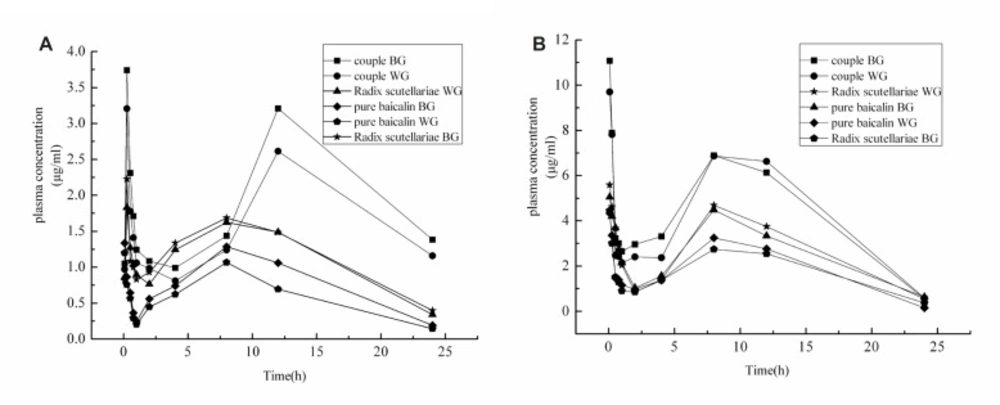
The mean plasma concentration-time profiles and standard deviation in normal rats and UC rats following the administration of pure baicalin, Radix Scutellariae or Scutellariae-Paeoniae couple extracts are depicted in Figure 3 (A) and 3 (B). Pharmacokinetic parameters resulting from non-compartmental analysis are listed in Tables 1 and 2.
| Parameter | Unit | Normal-rats | UC- rats | ||||
|---|---|---|---|---|---|---|---|
| Pure baicalin | Radix scutellariae | Scutellariae-Paeoniae couple | Pure baicalin | Radix scutellariae | Scutellariae-Paeoniae couple | ||
| Cmax | μg/mL | 1.53±0.22 | 2.49±0.27 | 4.18±0.42 | 4.42±0.06* | 5.84±0.47* | 11.05±0.53* |
| Tmax | h | 0.087±0.02 | 0.27±0.03 | 0.28±0.03 | 0.11±0.7 | 0.16±0.11 | 0.12±0.1 |
| AUC0-t | μg/mL·h | 17.77±0.66 | 28.04±4.06 | 49.01±4.61 | 41.46±0.62* | 59.12±6.42* | 104.87±0.86* |
| T1/2 | h | 7.15±1.58 | 7.35±0.76 | 9.76±1.64 | 7.04±0.37 | 7.68±0.39 | 11.96±0.61 |
| MRT | h | 9.46±0.51 | 9.73±0.35 | 10.25±0.22 | 9.36±0.17 | 9.62±0.27 | 12.64±0.58 |
| Parameter | Unit | Normal-rats | UC- rats | ||||
|---|---|---|---|---|---|---|---|
| Pure baicalin | Radix scutellariae | Scutellariae- | Pure baicalin | Radix scutellariae | Scutellariae-Paeoniae couple | ||
| Cmax | μg/mL | 1.23±0.12 | 1.79±0.23 | 4.12±0.15 | 4.42±0.26* | 5.32±0.34* | 10.06±0.94* |
| Tmax | h | 0.11±0.1 | 0.22±0.09 | 0.21±0.08 | 0.12±0.08 | 0.14±0.09 | 0.15±0.1 |
| AUC0-t | μg/mL·h | 14.67±1.93 | 22.89±3.17 | 40.35±4.96 | 40.86±5.31* | 57.95±1.91* | 101.31±3.05* |
| T1/2 | h | 8.20±0.42 | 8.80±0.95 | 10.88±0.51 | 8.99±0.94 | 9.79±0.32 | 11.76±1.89 |
| MRT | h | 9.74±0.08 | 9.48±0.48 | 11.6±0.73 | 9.46±1.32 | 11.75±1.67 | 12.52±0.87 |
Following oral administration of pure baicalin (purity > 95.5%) to rats, wogonoside was detected in all rats plasma in addition to baicalin, the plasma concentration was almost equal to that of baicalin. The same result was observed after oral administration of Radix scutellariae extract (200 mg/kg of baicalin, 28 mg/kg wogonoside) or Scutellariae-Paeoniae couple extract (200 mg/kg of baicalin, 27 mg/kg of wogonoside) to rats. The present result suggested that baicalin might convert to wogonoside in the rat body. Zhenyu Zhu et al. (4) reported that they found a peak (named M) with a retention time of about 5 min at m/z 461 was similar to wogonoside (m/z 461) after oral administration of Xiaochaihu Tang and Radix Scutellariae extract to rats, which was possibly a methylated product of baicalin. Methylation generally existed during the drug metabolism in-vivo, and due to the multiple hydroxyl groups on the baicalin benzene ring, the combination of a methyl group with a hydroxyl group was easy. So, we tentatively concluded that absorbed baicalin could be methylated to wogonoside in-vivo. Such a hypothesis, however, needs further substantiation.
Following oral administration of Radix Scutellariae or Scutellariae-Paeoniae couple extracts to rats, baicalein and wogonin with very high concentrations were presented in rat tissues (28), however, in the present study, it was below the detection limit in all plasma samples collected for all rats. These results appeared to be consistent with previous reports (14, 15, 29, 30). As described in previous studies, baicalin firstly must be hydrolyzed to baicalein by β-glucuronidase in-vivo, which was produced by both intestinal bacteria and intestinal epithelial cells (31–34). Thus, baicalin can first enter the intestinal wall as baicalein and is then reconverted to baicalin within the intestinal mucosa and thereby is absorbed into the blood. Similarly, baicalein is also converted to baicalin within the intestinal mucosa and absorbed. Therefore, baicalin in plasma may be derived from both baicalin and baicalein in Radix Scutellariae or Scutellariae-Paeoniae. The doses of baicalin and baicalein in the extracts were summed to produce the sum dose of baicalin and baicalin which was used to calculate dose-normalized Cmax and AUC0-t. Based on the similar chemical structure between wogonoside and baicalin, it is proposed that the transformation between wogonoside and wogonin are similar to that between baicalin and baicalein in the intestinal wall, the sum dose of wogonoside and wogonin was calculated in the same way. Consequently, we could only detect baicalin and wogonoside, not baicalein and wogonin in plasma.
As shown in Figure 3 and Table 1-2, the absorption speed of baicalin and wogonoside in the normal pure baicalin group was quicker than those in the normal Radix Scutellariae and Scutellariae-Paeoniae couple groups, Tmax for baicalin were: (0.087 ± 0.02) h, (0.27 ± 0.03) h and (0.28 ± 0.03) h; Tmax for wogonoside were (0.11 ± 0.02) h, (0.22 ± 0.09) h, (0.21 ± 0.08) h, respectively. But Tmax of baicalin and wogonoside were little different among the three groups of UC rats. The pharmacokinetic profiles differences of baicalin and wogonoside were reflected in AUC(0–t) and Cmax.
Following oral adminstration Scutellariae-Paeoniae couple extract to rats, Cmax and AUC(0–t) of baicalin and wogonoside were higher than the other two forms (p ≤ 0.05). Compared to the Radix Scutellariae groups, Cmax and AUC(0–t) of baicalin and wogonoside in the pure baicalin groups were significantly lower (p ≤ 0.05). Radix Scutellariae and Scutellariae-Paeoniae extracts contained many chemical components. These chemical components might produce certain pharmacological effects and have impact on the pharmacokinetic profiles of baicalin and wogonoside. Under the guidance of the theory of TCM, Radix Paeoniae alba, used as messenger herb and hematinic herb, could guide the bioactive ingredients of Radix Scutellariae to the proper tissues and exert a harmonizing effect, including the increase of absorption and bioavailability. Radix Paeoniae alba also could increase the absorption of baicalin and wogonoside through increasing the function of spleen and improving the patients’ digestive system vitality and immune system. Thus, compared to pure baicalin and Radix Scutellariae extract, the Cmax and AUC(0−t) of baicalin and wogonoside increased remarkably (p < 0.05) when Radix Scutellariae was mix-decocted with Radix Paeoniae alba, in contrast, the absorption of pure baicalin was the least. The present study demonstrated that herb extract could enhance the bioavailability of baicalin and wogonoside in rat plasma, a significant drug-drug interaction may occur and improve the absorption of baicalin and wogonoside when Radix Paeoniae alba is administered in combination with Radix Scutellariae (35).
Either in UC or in normal rats, baicalin and wogonoside demonstrated bimodal phenomenon in rat plasma after oral administration of pure baicalin, Radix Scutellariae or Scutellariae-Paeoniae couple extracts (Figure 3). The first absorption peaks occurred at about 0.15 - 0.27 h and the second was at about 8 – 12 h. The concentrations of the second peak (Cmax) and the values of AUC(0–t) dose were lower than which of the first peak. The ability of rapid absorption of baicalin and wogonoside may result in the rapid appearance of the first peak. Baicalin and wogonoside had double-site absorption kinetics, and baicalin and wogonoside undergo enteric circulation and enterohepatic circulation in rats after oral dosing, which may be responsible for the second peak. Baicalin and wogonoside were absorbed rapidly into the plasma and metabolized quickly. But they were always detected and kept their concentration at a certain range in plasma. It might be due to protein combination. Free drug might bind with plasma protein when it reaches a certain concentration (19). Wang et al. (36) reported that the binding rate of free baicalin with plasma protein could reach 80%. It was not able to maintain a high concentration for a long time because the baicalin would enter into the plasma and then bind with plasma protein. When the concentration of free baicalin decreased to a certain concentration, the bound baicalin would dissociate.
Comparative pharmacokinetics of baicalin and wogonoside after oral administration of pure baicalin, Radix Scutellariae and Scutellariae-Paeoniae couple extracts between UC and normal rats
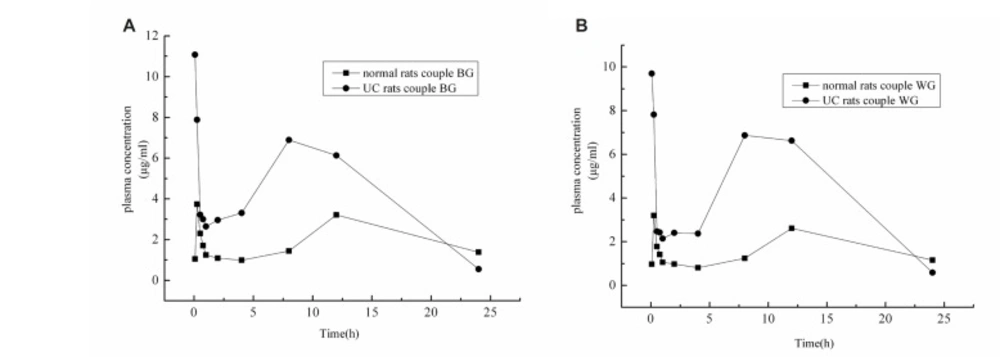
From Figure 4 and Table 1-2, it was noteworthy that UC rats showed better absorption than normal rats, regardless of oral administration of pure baicalin, Radix Scutellariae or Scutellariae-Paeoniae couple extracts. The absorption speeds of baicalin and wogonoside were very rapid, Tmax were approximately 0.13 h. Tmax showed no statistically significant difference among the three groups of UC rats. Cmax and AUC(0–t) of baicalin and wogonoside in UC rats were greatly higher than those in normal rats (p < 0.05). After oral administration of pure baicalin, Radix Scutellariae or Scutellariae-Paeoniae couple extracts to rats, AUC(0–t) of baicalin were: (41.46 ± 0.62), (59.12 ± 6.42) and (104.87 ± 0.86) (μg/mL)·h in UC groups vs (17.77 ± 0.66), (28.04 ± 4.06) and (49.01 ± 4.61) (μg/mL)·h in normal groups; AUC(0–t) of wogonoside were: (40.86 ± 5.31), (57.95 ± 1.91) and (101.31 ± 3.05 )(μg/mL)·h in the UC groups vs (14.67 ± 1.93), (22.89 ± 3.17) and (40.35 ± 4.96) μg/mL/h in the normal groups, respectively.
The significantly higher Cmax and AUC(0–t) of baicalin and wogonoside in UC rats indicated that ulcerative colitis had an influence on the pharmacokinetic characteristics of baicalin and wogonoside in pure baicalin, Radix Scutellariae or Scutellariae-Paeoniae couple extracts. Several factors were likely to be involved in the alteration of the pharmacokinetic behavior of baicalin and wogonoside under the colitis condition, including β-glucuronidase, UDP-glucuronosyltransferase, MRP2, intestinal or hepatic metabolic enzymes and bile flow rate, etc. (37, 38, 39). Trinitrobenzene sulfonic acid (TNBS)-induced UC condition might alter the formation of glucuronide, glutathione, and sulfate conjugates which played an important role in the metabolism of compounds in rats (40-43). These factors may result in a higher exposure of baicalin and wogonoside after oral doses. As known that intestinal flora plays an important role in the absorption and reabsorption of baicalin and wogonoside, we hypothesized that intestinal bacteria in UC rats would be very rich which lead to increased β-glucuronidase activity. Thus, profound intestinal β-glucuronidase activity due to the ulcerative colitis condition may have enabled the rapid conversion of baicalin and wogonoside to baicalein and wogonin which have the propensity to be absorbed more completely leading to higher baicalin and wogonoside exposure after oral administration relative to normal rats.
The findings of this study demonstrated that the pharmacokinetic behaviors of baicalin and wogonoside were remarkably altered in UC rats. Scutellariae-Paeoniae couple extract may exert more effectiveness than pure baicalin or Radix Scutellariae extract. It proves the rationality of using Scutellariae-Paeoniae couple for the treatment of ulcerative colonitis disease.
Conclusions
In summary, the results of the present study highlighted that drug–drug interactions, herb–drug interactions and herb–herb interactions always existed and affected the pharmacokinetic behavior of herbal ingredients. Statistically significant differences (p < 0.05) in pharmacokinetic parameters of baicalin and wogonoside including Cmax and AUC(0–t) were obtained among the rats orally administered pure baicalin, Radix Scutellariae or Scutellariae-Paeoniae couple extracts. The huge number of active ingredients in TCM made them suitable for multi-target actions. Thus the multiherb remedy might be a reasonable prescription [44]. Ulcerative colitis could influence the potential pharmacokinetic of baicalin and wogonoside. Baicalin and wogonoside had a better absorption effect, which would improve the therapeutic efficacy. We need to take caution when extrapolating PK and exposure data from healthy animals to diseased animals in designing pharmacological studies.