Introduction
The concept of bioactive glass (bioglass) was developed and examined for the first time by Hench at the beginning of the 70s (1, 2). Bioactive glass is a bone substitute material that is thought not only to have osteoconductivity, but is also responsible for osteoproduction by stimulating proliferation and differentiation of osteoprogenitor cells through a direct genetic control (1, 3-5). The discovery of this new material led Hench and Wilson to propose the concept of “osteostimulation” or “osteopromotion” to define this class of bioactive material and its effect on the genetic activation of bone cells (6). Bioactive glass is a surface reactive material that, when in contact with physiological fluids, releases soluble ionic products and induces insulin-like growth factor II mRNA expression and protein synthesis that have been suggested to stimulate in-vitro osteogenesis (4, 7). In addition in-vivo studies have demonstrated beneficial results from their use in various clinical situations (8-11). They are now clinically approved foruse in dense form in non-load bearing applications such as middle ear prosthesis and endosseous ridge implants and as a particulate for periodontal defect repair (12-13).They have potentials as bone replacement graft materials and have effectiveness as an adjunct to intrabony defects surgical treatment (12-14). Recent investigations have suggested that bioactive glasses have a much better performance in bone tissue engineering than hydroxyapatite (HA) (4, 15-17). After implantation, interaction with surrounding tissues results in a time-dependent alteration of the materials’ surface and the formation of a hydroxyl carbonate apatite layer that is very similar to the mineral phase of bone (2). Results of in-vivo implantation show that these compositions produce no local or systemic toxicity, no inflammation and no foreign-body response and possess antimicrobial properties (18, 19).
It seems that nanostructure bioceramic has better bioactivity compared to coarser crystals (20, 21). By controlling the structure and particle size in the range of nanoscale, some properties of bioactive glass such as osteoconductivity, sintering characters, solubility and mechanical reliability can be improved (22). The biocompatibility and cytotoxicity of the novel biomaterials is a key issue that should be addressed prior to pre-clinical applications. Thus, the aim of this study was to evaluate and compare the cytotoxicity of a nanopowder bioactive glass with a micropowder bioactive glass named NovaBone® as trade mark. The null hypothesis was that nanopowder bioactive glass will show an acceptable biocompatibility when compared to micropowder bioactive glass.
Experimental
Cells
Human HGF3-PI53 gingival fibroblast was obtained from the Pasteur Institute of Iran (Tehran). Cells were cultured in Roswell Park Memorial Institute (RPMI 1640, Sigma, USA) medium supplemented with 10% fetal bovine serum and 1 % penicillin/streptomycin antibiotic solution (10000 Unit/mL penicillin and 10 mg/mL streptomycin). Cells were incubated at 37°C with 5% CO2 and 95% humidity as reported previously (23-25).
Preparation of different bioglass concentrations
Micropowder bioactive glass (NovaBone®) as an FDA approved bone graft was purchased from US Biomaterials Corporation (Alachua, USA) with a particle size range of 90-710 μm containing 35.42 mol% SiO2, 57.44 mol% CaO and 7.15 mol% P2O5. A sol-gel derived nanopowder bioactive glass, with particle size mainly below 100 nanometers and containing 62.17 mol% SiO2, 28.47 mol% CaO and 9.25 mol% P2O5 were produced in Isfahan University of Technology (26, 27). Each bioactive glass was brought into suspensions with 0.5, 1, 1.5, 2, 5, 10, 15 and 20 mg/mL of medium. For achieving equilibration, these suspensions were placed into a shaker incubator for 48 h and afterwards were centrifuged for 10 min with 4000 rpm and the upper liquid was extracted. In order to sterilize the extract, it was filtered through a 0.2 μm microbiological filter.
Cell viability assay
Human gingival fibroblast cells were seeded (2×104 cells per well) in a 96-well plate and allowed to grow. After 24 h, different concentrations of bioactive glasses were added to the cells. The culture without extract was used as a negative control. Each condition was set up in triplicate. Plates were assayed 24 and 48 h after the addition of extracts. The 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay was used in this study to measure cell vitality and proliferation. The MTT agent reacts with its tetrazolium ring to produce blue formazan crystals in viable cells. At 24 and 48 h, the supernatant was removed from the wells, and the cells were rinsed three times with phosphate- buffered saline (PBS) to eliminate nonviable cells. 150 μL of fresh media was added to each well and then twenty micro liters per well of the MTT solution (5 mg/mL in PBS) was added, and the cells were incubated at 37°C for 4 h to allow the formation of formazan crystal. After incubation, the supernatant was removed, and 150 μL dimethyl sulfoxide was then added to each well to dissolve the formazancrystals under continuous pipetting. The optical density was read spectrophotometerically at a wavelength of 540 nm using microplate reader (Awareness, USA) and the percentage of living cells was calculated using the following formula:
Where blank absorbance was the media alone and the control absorbance was the cells with media alone. Cells without extracts were used as the reference for 100% cell survival. The results from three individual experiments were averaged and statistically analyzed using t-test and Spearman’s correlation coefficient. A p-value less than 0.05 was considered statistically significant.
Results
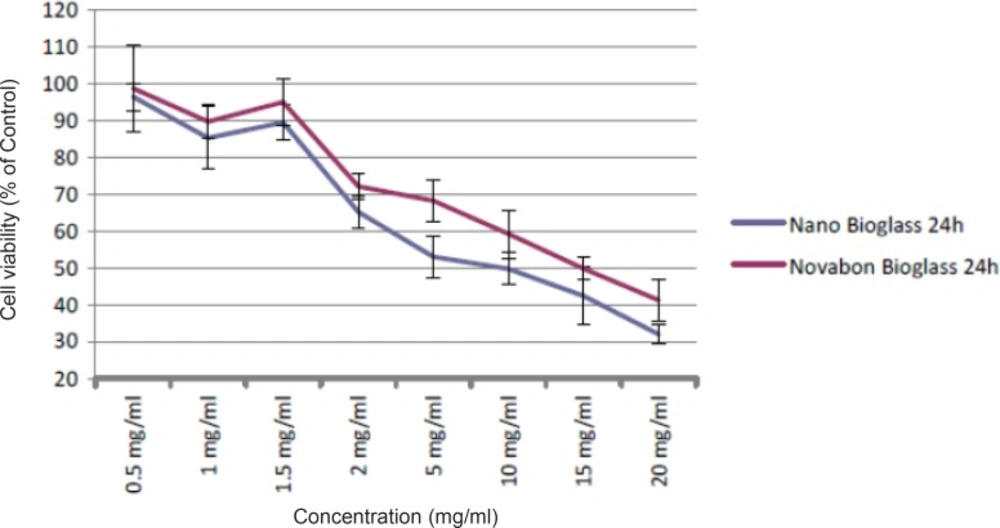
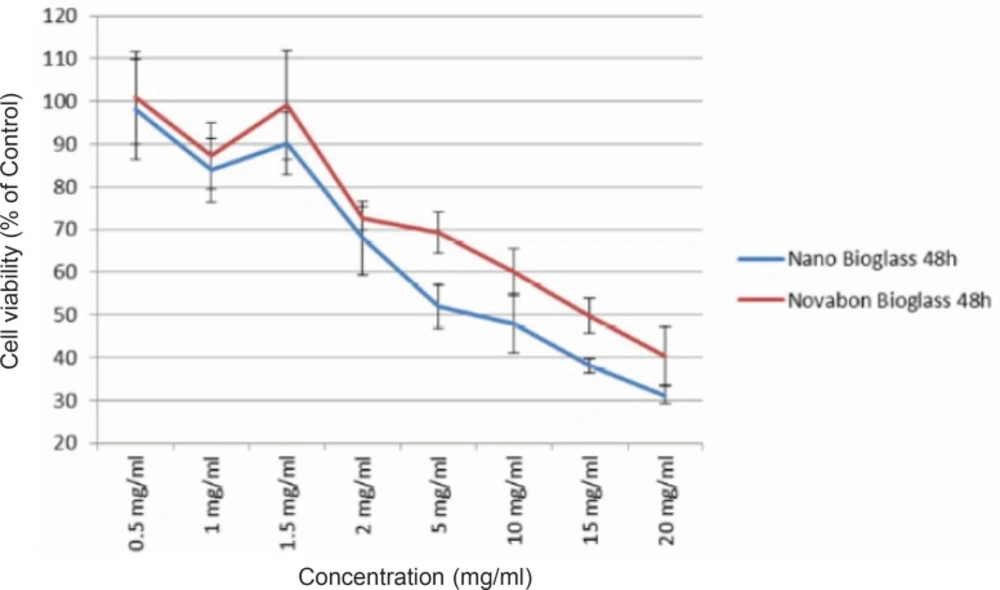
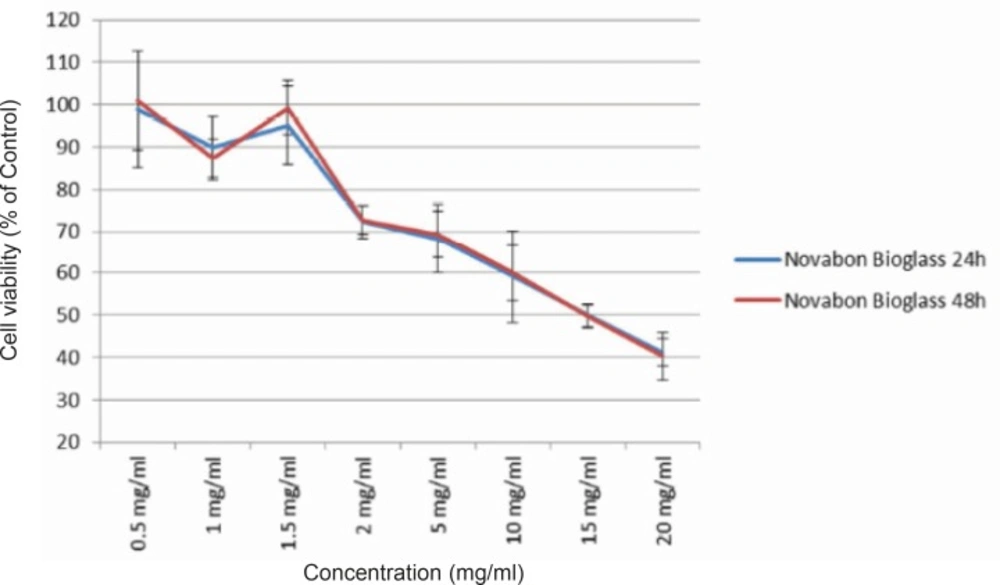
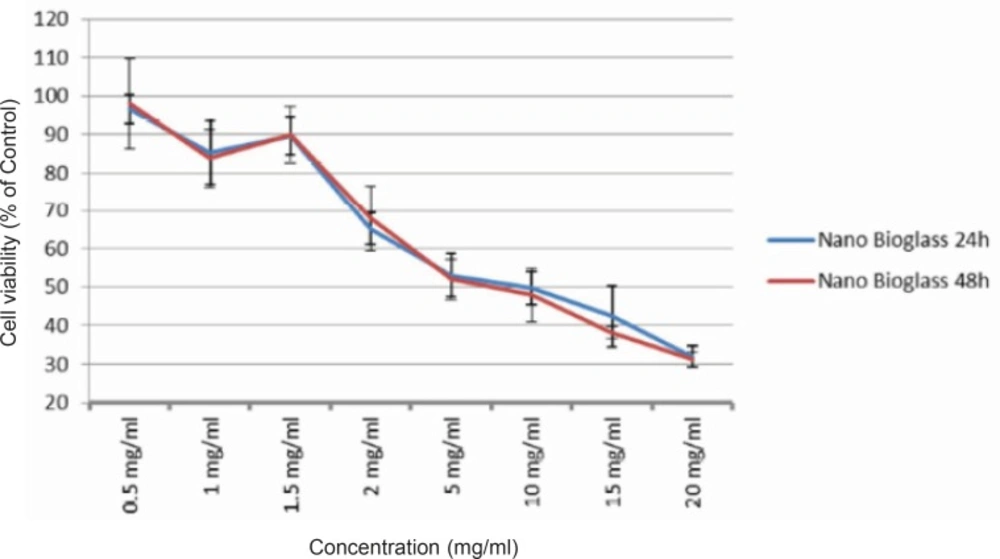
To detect changes in mitochondrial viability for gingival fibroblast cells incubated with different concentrations of two bioactive glasses tested, we measured the percentage of viable cells after 24 and 48 h using MTT assay (Figures 1 and 2). Statistically significant differences in cytotoxicity was found between the two bioactive glasses after 24 and 48 h. Two bioactive glasses had statistically significant differences only in 5, 10, 15 and 20 mg/mL concentrations (p-value < 0.05). Spearman’s correlation coefficient between concentration and cell cytotoxicity of micropowder and nanopowderbioglass were 0.941 and 0.951, respectively and there was a strong correlation between concentration and cytotoxicity in each group. As seen in Figures 3 and 4 there was no correlation between time and cell toxicity of the two bioactive glasses (p-value = 0.923 and 0.939 for micropowder and nanopowderbioglass, respectively using student t-test).
Viability of human HGF3-PI53 gingival fibroblasts exposed to different concentrations of Novabone and nanopowder bioactive glass after 24 h incubation. The cytotoxicity was determined by MTT assay. Data are expressed as the percentage of inhibition compared with negative control in which cell survival was assumed 100 % (mean ± SD, n = 9).
Viability of human HGF3-PI53 gingival fibroblasts exposed to different concentrations of Novabone and nanopowder bioactive glass after 48 h incubation. The cytotoxicity was determined by MTT assay. Data are expressed as the percentage of inhibition compared with negative control in which cell survival was assumed 100 % (mean ± SD, n = 9).
Viability of human HGF3-PI53 gingival fibroblasts exposed to different concentrations of Novabone bioactive glass after 24 and 48 h incubation. The cytotoxicity was determined by MTT assay. Data are expressed as the percentage of inhibition compared with negative control in which cell survival was assumed 100 % (mean ± SD, n = 9).
Discussion
The null hypothesis was accepted only for the concentration ≤ 2 mg/mL. Bioactive glass is a non-resorbable biomaterial andits medical use evolved three decades ago for its reported advantages of forming a strong bond with living tissues. Different concentrations of bioactive glass can be used in clinical applications. For example, a composition with 1.67 g/mL used for the treatment of hypersensitive teeth and even higher concentrations were used in the experiments by Allan et al. (28, 29). The biomaterials on the nano scale could stimulate reactions between the materials and the cells. For example, enhanced adhesion of osteoblasts has been observed on nanophase alumina, titanium and HA when compared to conventional bioceramics (30, 31). The use of nanopowder bioactive glass may increase particle solubility and enhance the release of such ions as calcium, phosphate and silica, thereby increasing pH level (26). Thus, the antimicrobial effects of bioactive glasses could be greatly enhanced by lowering their particle size, leading to their immediate release of alkaline species (32). Another advantage of reducing the particle size is higher resorption rate. Bioactive gel-glasses with higher silica contents had a slower rate of resorption, hence the resorption rate of bioactive glasses with high silica contents should be enhanced by reducing the particle size through a greater surface area of nanoparticles in contact with physiological fluids (26). Silicone is involved in the early stages of bone calcification, thus, the nanopowderbioglassmay be able to provide suitable conditions for the differentiation of osteoprogenitor cells to osteoblasts byreleasing proper amounts of silica in their surrounding in-vivo environment.
In this study, the in-vitro biocompatibility of two bioactive glasses was investigated via MTT assay. The results revealed that the presence of nanopowder bioactive glass atconcentration of 0.5, 1, 1.5 and 2 mg/mL did not affect viability of fibroblast cells, when compared with micropowder bioactive glass, but athigher concentrations, significantly less viability of cells was observed in nanopowder bioactive glass.
Doostmohammadiet al. used MTT assay to evaluate the cytotoxicity of 63S bioactive glass at theconcentrations of 5, 50, 100, 200 μg/mL on stem cells after 24, 72 and 96 h. They found that during the first day, cell proliferation was observed only in the culture under particle-free medium. Then, an increasing trend of cell number was measured during time in all cultures. After 72 h, cells cultured in particle-free medium showed the highest viability compared to that in other groups. However, after 96 h, there were no significant differences between the viability in the groups. They suggested that the initial inhibition of cell proliferation may be due to the adaptation of cells to the new growth medium (23). Mortazaviet al. evaluated the cytotoxicity of synthesized 58S, 63S and 72S nanopowders bioactive glasses using the sol-gel technique at theconcentrations of 6.25, 12.5, 25, 50 and 100 mg/mL on mouse fibroblast cells. The results showed that although 58S had some cytotoxic effects at 24 and 48 h incubation, it gavemore viability and proliferation by 72 h than the control. After 24 and 48 h, 63S and 72S showed no statistically significant differences with control group (26).
Smaller particle size results in the higher resorption rate and particle solubility. This may be responsible of more cytotoxicity in the first 48 h of application of nanopowders bioactive glasses compared with micropowdersatthe concentrations of more than 2 mg/mL in our study. These findings were not in agreement with Mortazaviet al. study which showed that 63S nanopowder bioactive glass at theconcentrations of more than 6.25 mg/mL did not affect the viability of mouse fibroblast after 24 and 48 h (26).Their study was conducted on mouse fibroblasts and this may havecontributed to the different results; thusmore studies are needed to compare the reaction of different cells to bioactive glass. There was no correlation between time and cell toxicity of two bioactive glasses assessed in this study. The results were not in agreement with Doostmohammadiet al. study (23). Although they used bioactive glasses in concentrations much less thanourstudies,cells may adapt more easily to the growth medium at lower concentrations.
The important point to be considered for the comparison of two bioglassesis the difference in their particle sizes. Compared to the microparticles, nanoparticles have about 1000 times more surface area and thus the amount and rate of ion release from nanoparticles are extremely higher. In other words, the bioactivity of nanoparticles is higher in comparison with the same mass of microparticles. Genotoxicity of nanoand micro bioactive glasses at the concentrations higher than 4 mg/mL has been evaluated in Tavakoliet al. study. They revealed that nanopowder glasses were more active in biological systems (33).
Conclusion
Based on the findings of this study, it can be concluded that cytotoxicity of nanopowder bioactive glass at concentrations ≤ 2 mg/mL is similar to micropowder bioactive glass at 24 and 48 h, however, it is more cytotoxic at concentrations ≥ 5 mg/mL in the first 48 h of application.