Introduction
Solid phase microextraction (SPME) was first introduced by Arthur and Pawliszyn (1). This method can incorporate sampling, extraction and sample presentation into a single step and become a useful method to most of the conventional analysis. Usually, a section of fiber, coated with a thin coating is used to extract the analytes and then the analytes sorbed on the fiber are desorbed and analyzed. The extraction efficiency of SPME is determined by the distribution of analytes between the matrix and the coating (2). As the fiber coating plays a key role in SPME, development of fiber coating for highly efficient extraction of the analytes of interest is an important research direction in SPME. Till now, a number of novel coatings have been developed and commercialized for the extraction of different kinds of compounds (2-4).
Carbon materials have long been used as absorbents for trapping or separation of organic compounds (5, 6). Carbon nanotubes (CNTs) are novel nanometer-sized materials with unique properties which have attracted much attention (7, 8). Generally, CNTs consist of two categories, single-wall carbon nanotubes (SWCNTs) and multiwall carbon nanotubes (MWCNTs), and have high electrical conductivity and relatively high surface area.
In recent years, composites of PANI and CNTs have attracted enormous interest due to their unique properties (9-11).
Palmitic acid or hexadecanoic acid is one of the most common saturated fatty acids found in animals and plants. Palmitic acid is the first fatty acid produced during lipogenesis (fatty acid synthesis) and from which longer fatty acids can be produced. It has the formula CH3(CH2)14COOH. Oleic acid or 9-octadecenoic acid is a mono-unsaturated omega-9 fatty acid found in various animal and vegetable sources. It has the formula of CH3(CH2)7CH=CH(CH2)7COOH. Both of these acids exist in solvent organics as impurities. In the present work, we established a method for preparating PANI/CNT-coated platinum wire (SPME Fiber) and investigated their capability to extract palmitic acid and oleic acid from chloroform solution.
Experimental
Reagents and instrumentation
Palmitic acid and oleic acid were purchased from Sigma-Aldrich. Aniline and sulfuric acid were purchased from Merck (Darmstadt, Germany). Aniline was distilled and stored at 4ºC. MWCNTs were purchased from Iran Petroleum research center (Tehran, Iran).
Electrochemical experiments were conducted on a three-electrode polarograph system with a platinum wire as the working electrode, a platinum electrode as the counter electrode and a Ag/AgCl reference electrode. All the experiments were carried out at room temperature in a sulfuric acid solution (0.1 M).
SPME syringe was purchased from Azar electrode company (Iran) and GC-MS was used for identification of palmitic acid and oleic acid. Identification was accomplished by comparing the retention time and mass spectrum of the analyte with that of the reference compound. Commercially available Wiley and NIST libraries were also used for identification of palmitic acid and oleic acid.
Chromatographic system comprised of an Agilent GC-MS (6890 GC and 5973 MSD system, USA) equipped with HP-5 capillary column (30 m, i.d. 0.25 mm, film thickness 0.25 μm).
Carrier gas was helium at flow rate of 1.2 mL/min. Splitless injection system was used and oven temperature was started at 60°C, held for 0.5 min and increased to 280°C at 12°C /min and held for 30 min.
Electronic impact (EI) was used as ionization mode at 70 eV and mass range was 35-450 amu.
Preparation of SPME fibers
The platinum wires (0.2 mm diameter) were polished with a weighing paper, then rinsed with double- distilled water and cleaned in an ultrasonic bath.
Plane PANI coated fibers were prepared by electrochemical oxidation of aniline on the surface of a platinum wire. In brief a three-electrode system with a solution of 0.045 M of aniline and 0.1 M of sulfuric acid was used, and a 7.5 cm long platinum wire was mounted as working electrode (2.5 cm of the platinum wire was inserted into the solution). A platinum electrode as the counter electrode and a Ag/AgCl electrode as reference electrode were used.
PANI coating was deposited on the platinum wire by potential cycling between 0 and 1 V at scan rate of 0.05 V/s after 60 cycles.
The same system was used for the preparation of SPME fibers coated by PANI/CNT composite except that CNT was added into the solution at the amount of 4 μg CNT/mL prior to the start of polymerization process.
During the polymerization process, the surface of platinum wire turned into green-black color. After coating, the SPME fiber was washed by double distilled water several times to remove any remaining aniline or CNT particles. The fiber was then dried under a gentle nitrogen stream and conditioned for 24 h at 200°C in a vacuum over prior to its use.
SPME extraction procedure
Plane PANI and PANI/CNT SPME fibers were placed in standard palmitic acid and oleic acid in solution of chloroform (0.05, 0.5 and 1 μg/mL) for 1 h. During extraction the temperature was maintained at 25°C with stirring the solutions. The fibers were then withdrawn and dried under a stream of N2 gas for 30 min. A SPME syringe was used to insert the fibers into the injection port of GC.
Results and Discussion
PANI can be deposited on platinum wire by electropolymerization of aniline in an electrochemical cell. The quality of the polymer can be greatly influenced by reaction medium.
Sulfuric acid is the most suitable medium for electropolymerization of aniline. The optimum concentration of sulfuric acid was found to be 0.1 M in the presence of 0.045 M aniline in water.
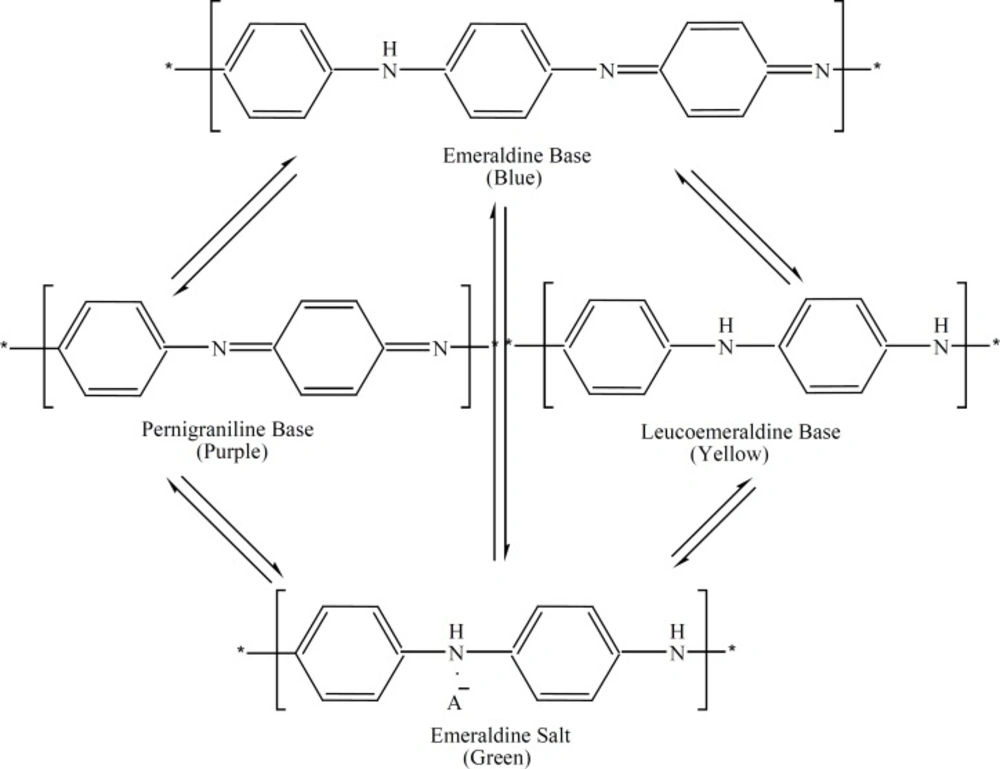
Figure 1 shows the different oxidation states of polyaniline (9, 12). The thickness of the coated film increases with increasing aniline concentration.
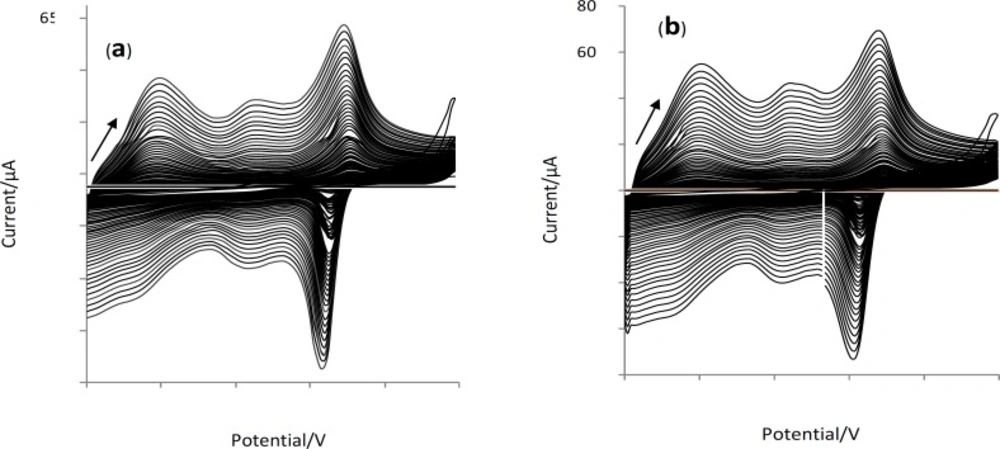
Cyclic voltammetry was employed as a highly convenient and reproducible method. The thickness of the coated film is controlled by the number of CV cycles. The best scan range was found to be 0 to 1 V. Electrochemical behavior of CNT-doped PANI composite and plane PANI coatings were monitored in 0.1 M H2SO4 through their voltammograms.
Figure 2 shows voltammograms obtained for the electrochemical deposition of PANI in sulfuric acid on the platinum wire both in absence (a) and presence (b) of CNTs. These two types of PANI coatings were used to extract palmitic acid and oleic acid from their chloroform solution for the concentrations within 0.05-1 μg/mL under the same conditions.
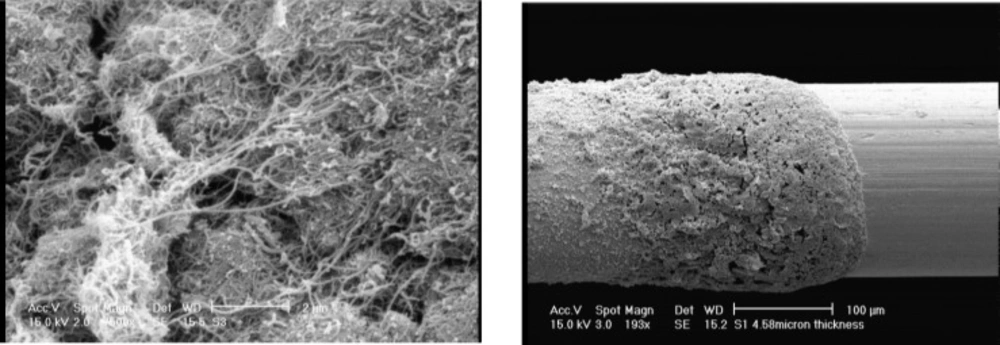
Figure 3 shows the scanning electron microscope (SEM) images of CNTs before being used in polymerization process (a) and film coating (PANI/MWCNTs composite) on the platinum wire (b).
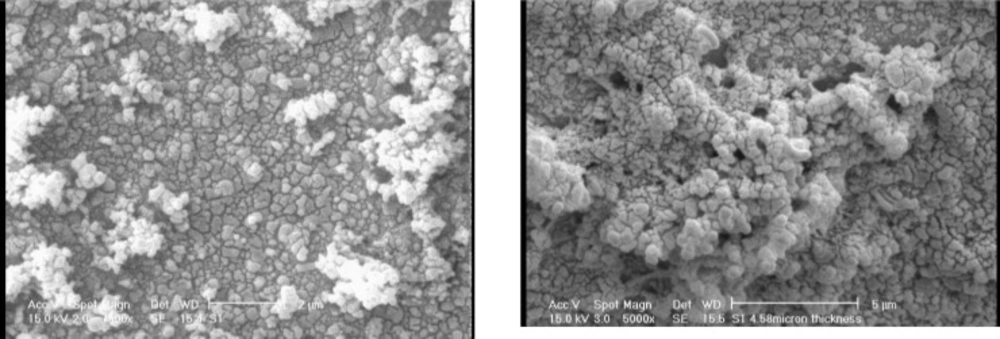
Figure 4 shows SEM Figure 4 shows SEM images of electrodeposited PANI (a) and electrodeposited PANI/MWCNTs composite (b). These images reveal that the two types of polymers have significant difference in the size and shape of polymer aggregates. This is in agreement with the larger extracting capacity of PANI/CNT composite fibers which has been observed for them in comparison to the plain PANI fibers.
Interestingly the higher peak current observed in voltammogram of PANI/CNT fibers also confirms the larger surface area for these fibers.
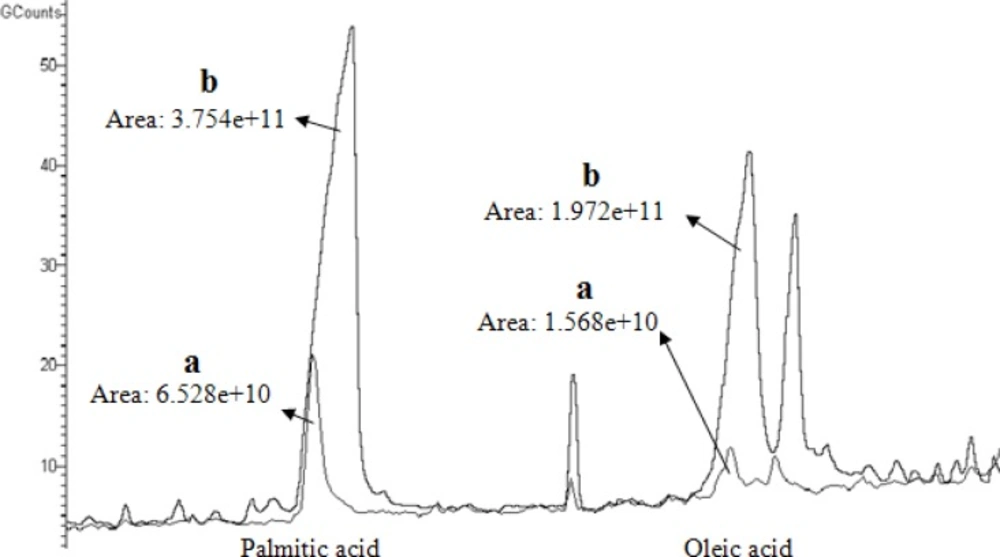
In Figure 5, the total ion chromatogram (TIC) obtained for palmitic acid and oleic acid after extraction from chloroformic solution by using plain PANI SPME is compared with the TIC obtained for the same compounds by using PANI/MWCNT SPME probes.
As it appears in Figure 5, both PANI coated and PANI/CNT coated probes had the ability to concentrate palmitic acid and oleic acid on their coating and produced strong signals in GC-MS chromatograms. In the meanwhile PANI/CNT coated SPME probes produced signals which were stronger than those produced by PANI coated SPME probes.
It could be suggested that the composite material with CNTs has both an increased surface area and an elevated adsorptive capacity which leads to this overall increase in extracted palmitic acid and oleic acid.
As it can be seen, CNTs are wrapped with PANI aggregates and it could be speculated that in the presence of CNTs, PANI grows around the CNTs and encapsulate them. This could be the reason for the greater capacity of PANI/CNT composites for extracting palmitic acid and oleic acid from their solutions.
In fact CNTs provide a scaffold for the deposition of PANI and this apparently leads to the formation of smaller aggregates and thus larger effective surface area for the absorption.
Conclusion
In conclusion, the present research revealed that CNTs could be used successfully in the preparation of SPME fibers and can greatly enhance the performance of these fibers. Electrochemical evidence as well as SEM images indicate that the presence of CNTs in the electrochemical cell while the process of polymerizaion is in progress causes the formation of polymers with larger surface area and thus more extracting capability.
In a more general sense, it could be concluded that the doping of electrochemically produced polymers for SPME with CNTs will enhance the adsorbing capacity of the resulted films. This could open a new horizon in the application of nanoparticles and nanotechnology in the field of SPME and analytical chemistry.
Further studies about the preparation of other SPME coatings in the presence of CNTs are being conducted.
In the meantime, the Stability of these types of polymers are being studied under different conditions in order to find out whether they are capable of being used for several times. Apart from being easily made and inexpensiveness, electrochemical preparation of CNT- doped coatings for SPME purposes is also quite flexible and the thickness of the fibers can be controlled by the number of CV cycles.