Introduction
Bone metastases are common in the progression of various tumors such as prostate, breast and lung carcinoma. They often entail an occurrence of progressive pain (1) and occur in many patients with solid malignant tumors (2). Approximately 50% of patients with breast carcinoma and 80% of patients with prostate carcinoma develop metastatic bone diseases and nearly half of them experience bone pain (3). Despite the treatment in these patients, a systemic bone avid radiopharmaceutical for the treatment of widespread bone metastases has potential benefits (4).
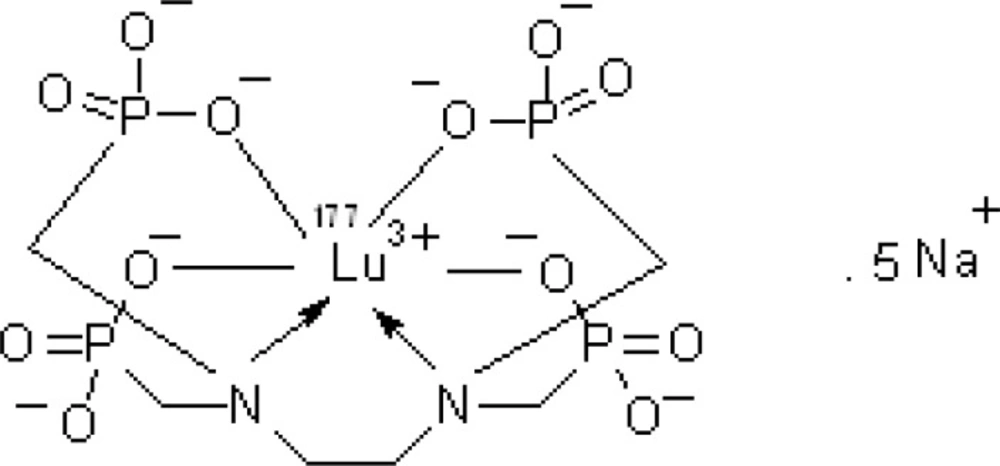
Multidentate polyaminopolyphosphonic acid ligands are known to form stable chelates with many metals including lanthanides. Among them, ethylenediaminetetramethylene phosphonic acid (EDTMP) can be envisaged as a possible carrier moiety for the development of beta emitter-based radiopharmaceutical for bone palliation.
Among radioactive lanthanides used in nuclear medicine, lutetium-177 (177Lu) is an interesting candidate for therapeutic protocols. 177Lu-radiopharmaceuticals have been developed and used in various procedures, such as somatostatin receptor radiotherapy (5), radioimmunotherapy (6), bone palliation therapy (7) and radiosynovectomy (8, 9).
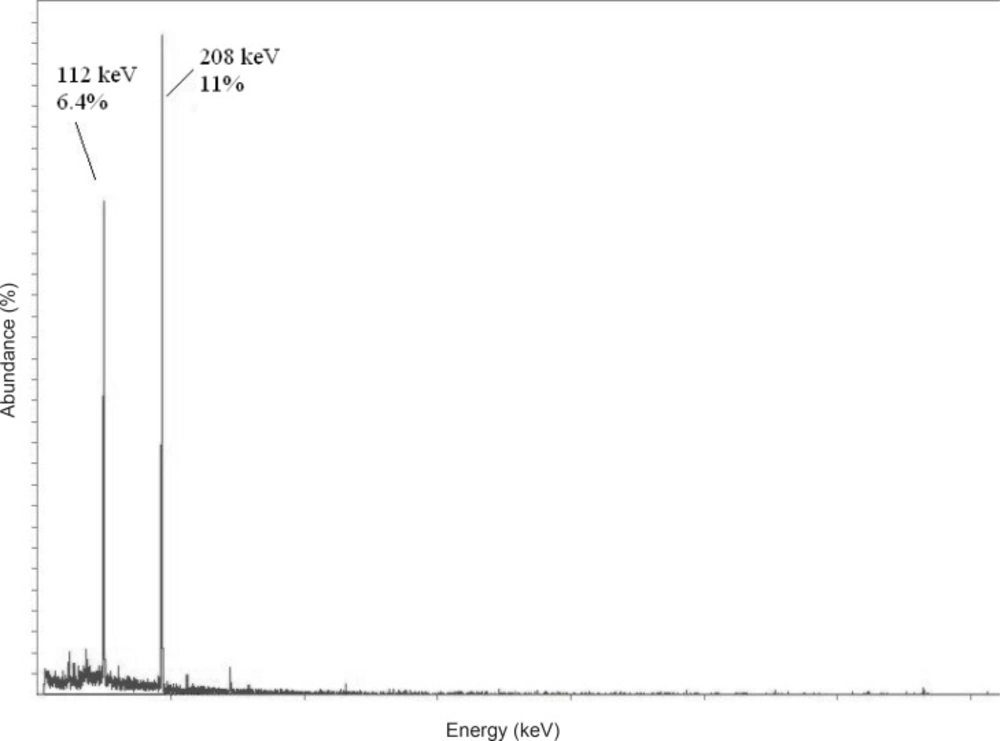
Due to the suitable decay characteristics [t1/2 = 6.73 days, Eβ max = 497 keV, E© = 112 keV (6.4%), 208 keV (11%)] as well as the feasibility of large-scale production in adequate specific activity and radionuclidic purity by using a moderate flux reactor, 177Lu is a promising radionuclide in the development of 177Lu-EDTMP complex as a therapeutic radiopharmaceutical agent (Figure 1).
Compared to Sm-153, Lutetium-177 has a longer half-life which provides the possibility of production of a radiopharmaceutical with better shipment quality and longer shelf-life. On the other hand, irradiation of biological targets by a nuclide with longer half-life may improve the effectiveness of bone pain palliation therapy.
In this research, Lu-177 ethylene-diaminetetramethylene phosphonic acid (177Lu-EDTMP) complex was prepared from in-house made starting ligand and the biokinetic studies of the compound were carried out in vital rat organs by post-mortem studies and imaging.
Experimental
177Lu was produced with a specific activity of approximately 70-80 mCi/mg and radionuclidic purity of 99.98% by irradiation of natural Lu2O3 (99.999% purity from Aldrich Co., UK) targeted at a thermal neutron flux of approximately 4-1013 n.cm-2.sec-1 for 5 days at Tehran Research Reactor (TRR). Whatman No. 3 paper was obtained from Whatman Company (UK). Radio-chromatography was performed by using a Bioscan AR-2000 radio TLC scanner instrument (Bioscan, France). A high purity germanium (HPGe) detector coupled with a Canberra™ (model GC1020-7500SL, Canberra Industries, Inc. CT, U.S.A.) multichannel analyzer and a dose calibrator ISOMED 1010 (Elimpex-Medizintechnik, Austria) was used for counting distributed activity in rat organs. All other chemical reagents were purchased from Merck Company (Germany).
Calculations were performed based on the 112 keV peak for 177Lu. All values were expressed as mean ± standard deviation and the data were compared using Student’s t-test. Animal studies were performed in accordance with the United Kingdom Biological Council’s Guidelines on the Use of Living Animals in Scientific Investigations (1987). The approval of NSTRI Ethical Committee was obtained for conducting this research.
The wild-type rats (NMRI), weighing 180-200 g, were purchased from Pasteur Institute of Iran (Karaj), and acclimatized at proper rodent diet and 12 h/12 h day/night light/darkness.
Production and quality control of 177LuCl3 solution
177Lu was produced by neutron irradiation of 1 mg natural Lu2O3, according to the reported procedures (10) at TRR (General Atomics, USA). The irradiated target was dissolved in 200 µL of 1.0 molL-1M HCl, for the preparation of 177LuCl3 and then diluted to the appropriate volume with ultra pure water for the production of a stock solution of final volume of 5 mL (0.04 molL1). The mixture was filtered through a 0.22 µm filter (Waters, USA) for the purpose of sterilization. The radionuclidic purity of the solution was determined for the presence of other radionuclides using purity germanium (HPGe) spectroscopy, through the detection of various interfering gamma emitting radionuclides. The radiochemical purity of the 177LuCl3 was evaluated using 2-solvent systems for instant thin layer chromatography (ITLC) [A: 10 mmolL-1 diethylenetriamine pentaacetic acid (DTPA), pH = 5 and B: 10% ammonium acetate:methanol mixture (1:1)].
Synthesis of EDTMP
EDTMP was synthesized from phosphorous acid, ethylenediamine and formaldehyde in the presence of HCl by a modified Mannich-type reaction (11). To the stirring mixture of phosphorous acid (33.66 g, 0.5 mole) and concentrated HCl (33.44 g) under N2 atmosphere, ethylenediamine dihydrochloride (5 g, 0.08 moles) was added drop wise and the mixture was heated to reflux. Aqueous solution of formaldehyde (37 %) was then added drop wise to the mixture. Reflux (at 100 °C) continued for 4 h and the boiling suspension was evaporated under vacuum. The residue was recrystallized from water/methanol mixture [m.p. 214-215°C; IR (KBr, ν cm-1): 3308, 2633, 2311, 1668, 1436, 1356; 1H-NMR (D2O, δ ppm): 3.53 (d, J = 12.3 Hz, 8H,-N-CH2-P=O), 3.85 (s, 4H, -N-CH2-); 13C NMR (D2O, δ ppm): 51.63, 52.73; 31P NMR (D2O, δ ppm): 10.52].
Radiolabeling of EDTMP with 177LuCl3
A stock solution of EDTMP was prepared by dissolving in 1 molL-1 NaOH and diluted to the appropriate volume with ultra pure water, in order to produce a solution of 50 mg.mL-1. For labeling, an appropriate amount of the 177LuCl3 solution (0.1 mL, 50 mCi) containing the required activity was added to the desired amount of EDTMP solution (0.3 mL, 1 to 5 mg.mL-1). The complex solution was then kept at room temperature for 60 min. The final solution was passed through a 0.22 µm membrane filter and the pH was adjusted to 7-8.5 with 0.05 molL-1 phosphate buffer (pH = 5.5). The radiochemical purity was determined using Whatman 3 MM chromatography paper or ITLC-SG, eluted with NH4OH (56%): methanol (%100): water (%100) (0.2:2:4; v/v/v) mixture.
Sterility and Apyrogenicity of the radiopharmaceutical
Sterility was controlled on a random sampling following the decay of radioactivity. The Limulus Amoebocyte Lysate (LAL) test was used for the validation of radiopharmaceutical production method according to European protocol (12).
Stability of 177Lu-EDTMP in final formulation
Stability of 177Lu-EDTMP in the final preparation was determined by storing the final solution at 25 °C for 2 days and performing frequent ITLC analysis for the determination of radiochemical purity using Whatman 3 MM chromatography paper or ITLC-SG, eluted with NH4OH (56%):methanol (%100):water (%100) (0.2:2:4; v/v/v) mixture.
Stability of 177Lu-EDTMP in presence of human serum
Final 177Lu-EDTMP solution (200 µCi, 50 µL) was incubated in the presence of freshly prepared human serum (300 µL) (Purchased from Iranian Blood Transfusion Organization, Tehran, Iran) at 37 °C for 2 days. The stability was determined by performing frequent ITLC analysis using the above-mentioned chromatography system.
Biodistribution studies
The biodistribution of free Lu3+ cation as well as of 177Lu-EDTMP was determined in wild-type rats. For each radiochemical compound, 100 µL (150 µCi) radioactive solution was injected directly to normal rat through caudal vein. The animals (n = 3) were sacrificed at predetermined times following the injection (2 h to 7 days) and the percentage of the injected dose in the tissues was then determined with a ©-ray scintillation or a dose calibrator.
Single photon emission computed tomography (SPECT) imaging of 177Lu-EDTMP in wild-type rats
For imaging studies, 177Lu-EDTMP solution (7.4 MBq, 200 µL) was injected intravenously to rats through their tail veins, followed by the injection of propofol-xylazine mixture for anaesthetization. The images were taken after administration of the radiopharmaceutical by a single-head SPECT system (Siemens, Germany) based on 112 keV peak (15% energy window). The rat-to-septa distance was 12 cm.
Results and Discussion
The radionuclide was prepared at a range of specific activity of 3 to 5 MBq.mg-1. For radiolabeling use, after counting the samples on an HPGe detector for 5 h, two major photons (6.4% of 0.112 MeV and 11% of 0.208 MeV) were observed (Figure 2).
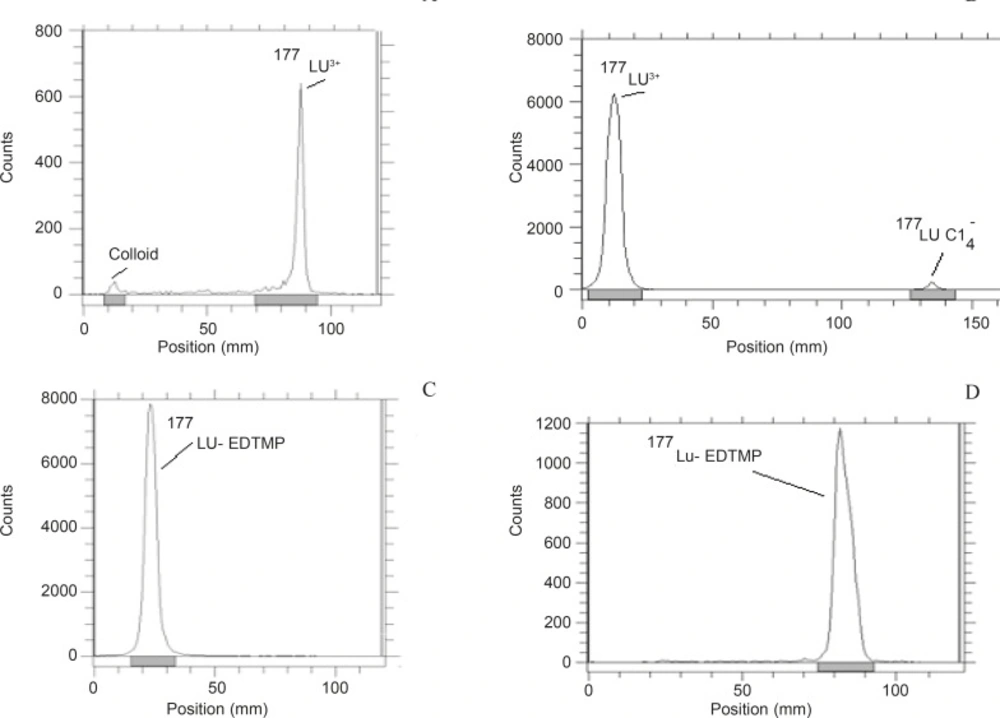
The radiochemical purity of the 177Lu solution was determined in two solvents. In 10 mmol.L-1 DTPA aqueous solution (solvent 1), free Lu3+ cation is complexed to more lipophilic Lu-DTPA form and migrates to higher Rf. Small radioactive fraction remaining at the origin could be related to other Lu ionic species and not due to the forming of Lu-DTPA complex (such as LuCl4-, etc.) and/or colloids. On the other hand, 10% ammonium acetate:methanol mixture (1:1) (solvent 2) was also used for the determination of radiochemical purity. It seems that the fast eluting species was possibly Lu3+ and other ionic forms of 177Lu (such as LuCl4-) remained at the origin (Rf = 0) as well as colloids (Figure 3). The differences between the impurity peaks in the two chromatograms could be related to the presence of colloidal impurity (2%). Nearly, 2% of activity can also be attributed to other ionic impurities. The effect of various factors on the labeling yield of 177Lu-EDTMP including ligand concentration and temperature were also studied and the corresponding results are shown in Table 1.
| Lu:EDTMP molar ratio | Radiochemical yield (%) |
|---|---|
| 1:5 | 98.2 |
| 1:10 | 99.2 |
| 1:15 | 99.7 |
| 1:20 | 99.5 |
| 1:40 | 99.4 |
As observed, labeling yield increased with increasing molar ratio of Lu:EDTMP (from 1:5 to 1:15) and reached more than 99% in 60 min.
The stability of prepared 177Lu-EDTMP complex was studied up to 48 h after preparation. The complex was found to be stable in final pharmaceutical sample and its radiochemical purity was calculated above 99% even 48 hours after the preparation by using Whatman 3 MM eluted with NH4OH: methanol: water (0.2:2:4) mixture. Stability test was also developed for the complex in presence of human serum at 37 °C using ITLC as mentioned above.
ITLC chromatograms of 177LuCl3 solution in a DTPA solution (pH = 5). (a) 10% ammonium acetate:methanol (1:1; v/v) solution, (b) chromatograms for 177Lu-EDTMP eluted using 10% ammonium acetate:methanol (1:1; v/v) solution, (c) chromatograms for 177Lu-EDTMP eluted and with NH4OH: methanol:water (0.2:2:4) mixture, (d) chromatography with Whatman 3 MM
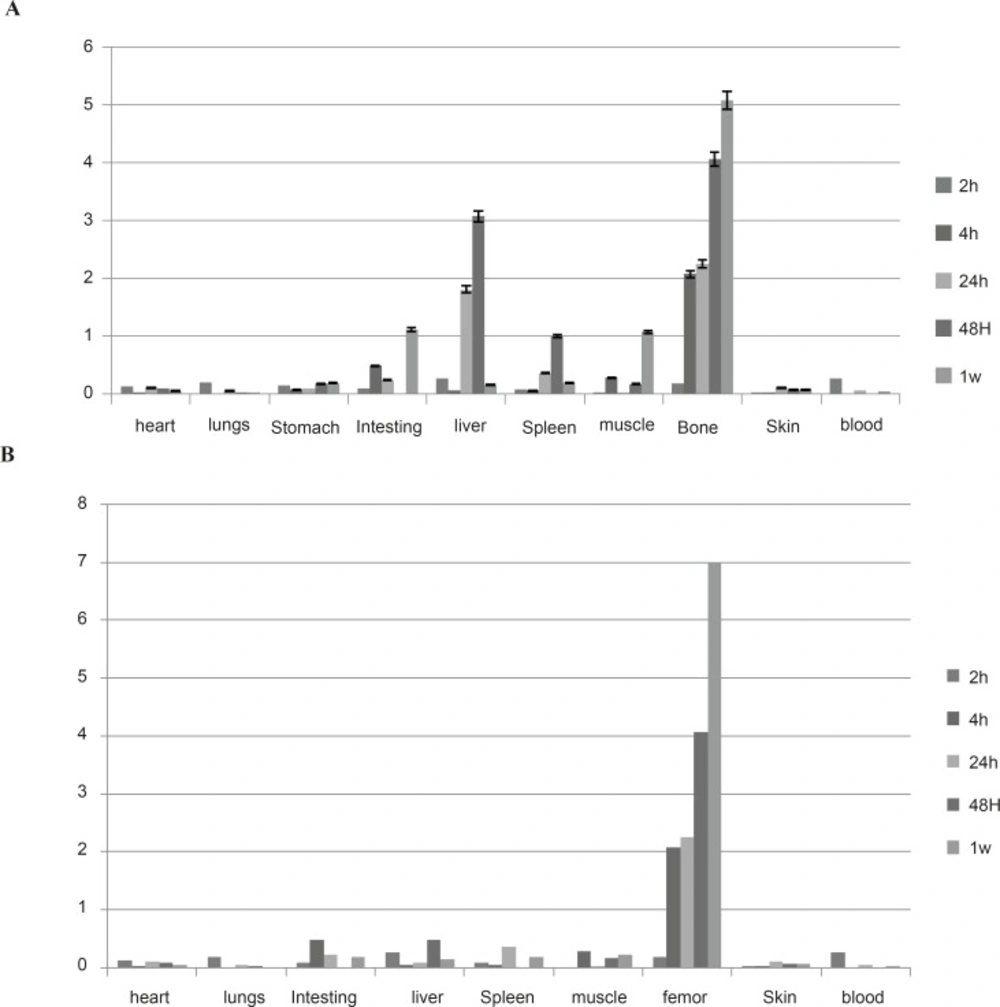
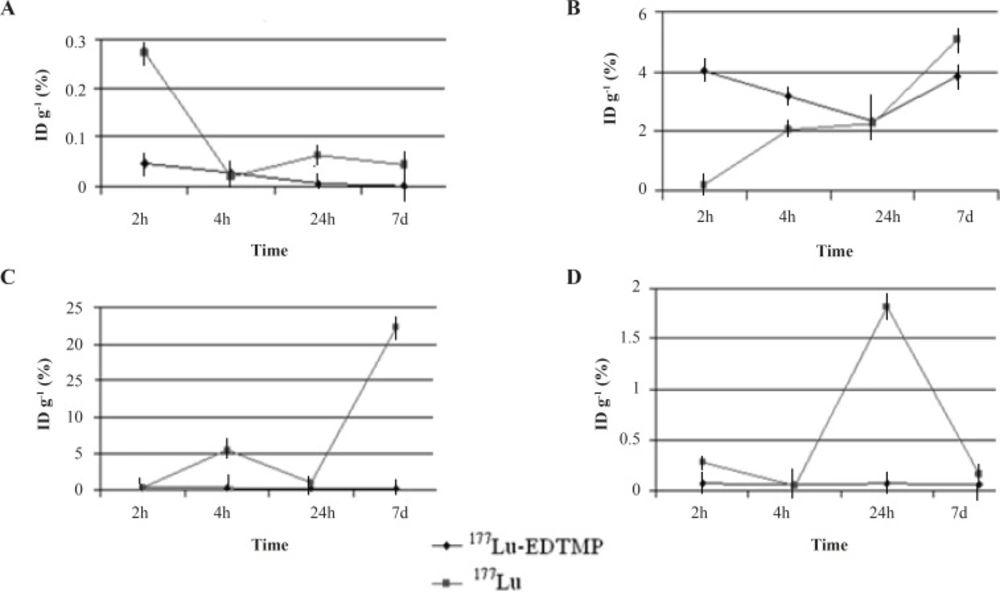
Regarding the animal studies, the tissue uptakes were calculated as the percent of area under the curve of the related peak per gram of tissue (% ID/g) (Figure 4).
The biodistribution of 177Lu cation was determined in wild-type animals for 2-48 h post injection. The liver radioactivity uptake of the cation is comparable with other radio-lanthanides such as Yb, Sm and Tb (13). About 3% of the cation radioactivity accumulate in the liver in 48 h.
Low blood radioactivity content demonstrates the rapid removal of 177Lu from the circulation after injection. Lung, muscle and skin did not uptake the 177Lu significantly, while it is in accordance with other cations accumulation. A 4% bone uptake was observed for 177Lu which remained almost constant after 48 h (data not shown). Significant uptake of 177Lu uptake was also observed in the spleen, possibly related to reticuloendothelial system. Kidney plays an important role in the excretion of 177Lu as a water soluble cation especially after 24 h. The radioactivity biodistribution of 177Lu-EDTMP (200 µCi in 150 µL volume) in rat organs up to 7days post injection was determined and it was clearly observed that the major portion of injected radioactivity of 177Lu-EDTMP was transferred from blood circulation into bones (Figure 4).
Figure 5 compares the 177Lu-EDTMP and 177LuCl3 species behavior in biodistribution and demonstrates the blood accumulation from 2 h to 7 d. Both compounds were washed out from the circulation after 4 h.
177Lu-EDTMP was rapidly taken up in bones within 2 h after administration and radioactivity concentration retained almost constant up to 24 h. The accumulation increased slowly after 7 days, whereas the uptake of free 177Lu increased slowly. On the other hand, 177Lu-EDTMP showed more uptakes at all time intervals compared to free cation, except after 24 h. The error bars at this time point have overlaps, demonstrating the possibility of higher bone uptake even at this stage.
Rapid 177Lu-EDTMP uptake and low blood circulation activity lead to a low kidney excretion, whereas 177Lu3+ is washed out through kidneys in 7 days as predicted for a cationic species.
Unlike 177Lu3+ which accumulates significantly in the liver, 177Lu-EDTMP has almost no liver accumulation, which is a major advantage of a therapeutic radiopharmaceutical due to the possibility of increasing the maximum injectable dose.
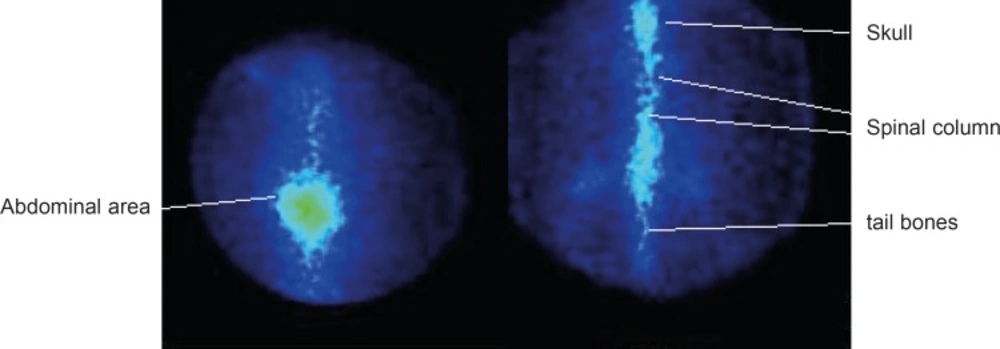
As shown in Figure 6, the complex is mainly washed out from the circulation in the first few hours and is trapped in bone tissues especially in spinal column, skull tail and thigh bones. Insignificant activity is accumulated in other tissues. In case of free 177Lu, most of activity is accumulated in liver and abdominal area GI.
Recently a similar report on the production and quality control of 177Lu-EDTMP complex has been reported (14) demonstrating higher localization of 177Lu-EDTMP in skeleton compared to 153Sm-EDTMP which is also in agreement with our data.
In a recent study on the species-specific pharmacokinetic behavior of the drug in canine model did not demonstrate any biological adverse effects suggesting 177Lu-EDTMP as a promising radiopharmaceutical for further human studies (15).
As a comparison, the labeling and QC of the 177Lu-EDTMP complex was more or less similar to the Sm-153 analog and no significant difference was observed in the biodistribution data. The radiopharmaceutical higher bone uptake and half life may impose long term bone marrow and other critical organs irradiation. Thus, theoretical and practical dosimetry studies should be considered for this ligand at various injected doses to humans.
Conclusions
EDTMP ligand was synthesized and its structure was determined using authentic spectroscopic methods. 177Lu-EDTMP was prepared (radiochemical purity more than 99%) using optimization studies. 177Lu-EDTMP and 177LuCl3 preparations were administered intravenously through the tail vein to wild-type rats and biodistribution data collected after 2 h to 7 days showed at least 70% accumulation of the drug in the bone tissues. SPECT images were taken from wild-type rats injected with 177Lu-EDTMP and 177LuCl3 after 24 h and the biodistribution was shown to be consistent with post-mortem data. A comparative accumulation study for 177Lu-EDTMP and 177Lu was performed for vital organs up to 7 days. 177Lu-EDTMP seemed a promising agent for bone pain palliation therapy in skeletal metastases in humans. Thus the radiopharmaceutical was released for clinical trial process in 20 volunteer human with metastatic bone malignancies in Shiraz, a central city in Iran (16).