Introduction
Antipsychotics which are routinely used in the management of schizophrenia and other affiliated disorders are often associated with distressing extra-pyramidal side effects. The phenomenon of cataleptic immobility-induced in animals by typical neuroleptics (e.g. haloperidol) is a robust behavioral model to study the nigrostriatal function and its modulation by cholinergic, dopaminergic and other neurotransmitter systems (1). Haloperidol-induced catalepsy occurs due to the blockade of dopamine (D2) receptors and reduced dopaminergic transmission. Enhanced stimulation of the intrinsic central cholinergic system has also been implicated in haloperidol-induced catalepsy as it has been reported to be enhanced and antagonized by pilocarpine and the cholinergic blocker, atropine, respectively (1).
Interesting evidences suggest the important roles of inflammatory reactions accompanied with the pathological processes caused by cyclooxygenase-2 (COX-2) seen in many neurodegenerative disorders, including Parkinson’s disease (PD) (2, 3). Furthermore, the inhibition of COX-2 or COX-2 gene expression as we previously showed can improve the movement disorders of PD in animal model (4-6). Also previous reports suggest that COX-2 can cause an increase in the level of acetylcholine in the brain through producing the prostaglandin E2 and increasing the expression of cholinergic markers, such as choline acetyltransferase and vesicular acetylcholine transporter protein. It has been mentioned that prostaglandins have modulatory effects on adrenergic, noradrenergic and glutaminergic transmissions (7, 8). In addition, some of the investigations have shown that COX-2 inhibitor impairs the spatial memory through the reduction of acetylcholine level in the brain (9, 10).
The compound 11b [1-(phenyl)-5-(4-methylsulfonylphenyl)-2-ethylthioimidazole] (as we reported (11) its synthesis and biological potencies as the most potent and selective COX-2 inhibitor (COX-2 IC50 = 0.58 μM with no inhibition of COX-1 up to 25 μM) relative to the reference drug celecoxib (COX-2 IC50 = 0.21 μM with no inhibition of COX-1 up to 25 μM)) was selected to investigate its effects on the haloperidol-induced catatonia as a different PD animal model and also neuroleptic overdose animal model. Furthermore, simultaneous to the catalepsy measurement, the in-vivo assay of dopamine concentration changes in the striatum as the affecting area in the cataleptic disorders after the administration of selective COX-2 inhibitor, was the latter interest of this research.
Experimental
Animals
Adult male albino rats (weighing 250-300 g) were selected for the study. The animals were purchased from Pasteur Institute of Iran and housed in stainless steel cages, handled daily, and provided with food and water ad libitum. A 12 h light/12 h dark cycle was maintained and animals were tested during the light cycle. These animals’ experiments were carried out in accordance with the recommendations from the declaration of Helsinki and the internationally accepted principles in the use of experimental animals.
Chemicals
Compound 11b was prepared as we previously described (10). Scopolamine and haloperidol were purchased from Merck (Merck, Germany). Compound 11b and haloperidol were freely dissolved in distilled water and scopolamine was dissolved-suspended in 1% Gum acacia solution. In acute study, all injections were IP and in chronic, all injections were P.O except the haloperidol IP.
Surgery and microdialysis procedure
After anesthetizing [75 mg/Kg ketamine combined with 8 mg/Kg Xylazin IP] and placing the rats in the stereotaxic apparatus, a sagittal incision was made in the scalp with sterile blade. Subsequently, the skin and inferior tissue layers covering the skull were retracted and then, the skull was exposed and a hole was drilled through it in the area overlying the right striatum, using the following coordinates with respect to the bregma: A/P + 1 mm; M/L + 3 mm, D/V + 6 mm according to the atlas (12). A guide-cannula lowered into the brain for inserting the microdialysis probe which delivered a modified Ringer solution through the probe, was fixed to the cranium and the incision was closed. Surgery was performed using sterile instruments and aseptic conditions. Rats were allowed to recover from the surgery for 7-10 days. On the experimental day, a microdialysis probe was inserted into the cannula, and the inputs of the probes were connected to a microperfusion pump, CMA/102 infusion pump (CMA/Microdialysis, Sweden), which delivered a modified Ringer solution (147 mM NaCl, 1.2 mM CaCl2, 2.7 mM KCl, 1.0 mM MgCl2 and 0.04 mM ascorbic acid) through the probe at a flow rate of 2 μL/min. Ringer solution was then infused for 3-3.5 h before the baseline samples being collected to obtain stable basal extracellular levels of dopamine. The microdialysate samples (20 μL) were collected every 20 min. When a stable outflow was shown by four consecutive samples of neurotransmitters, rats were given orally Compound 11b (2, 4 and 8 mg/Kg) and Scopolamine (1 mg/Kg) and Dimethyl Sulfoxide (DMSO) as vehicle. Control rats received a saline injection (1 mL/Kg). The dialysates were collected for 4 h after the administration of drugs-vehicle. The stress caused by the IP vehicle injection and handling of the rats was not found to alter the extracellular glutamate-dopamine levels. In part of experiments, when the rats were given drugs or vehicle after four stable consecutive samples, the dialysates were collected for 2.5 h after the injection. Microdialysate levels of dopamine were analyzed immediately. After the experiments, the location of the probe was determined histologically on serial coronal sections. Only data obtained from rats with correctly implanted probes were included in the results. All experiments were made with awake, conscious animals. Animals were individually housed for the duration of the experiment in a CMA/120 system (CMA/Microdialysis, Sweden). This procedure was performed at the 1st and the 7th day of the study. Dopamine samples were analyzed by the reverse-phase, High Performance Liquid Chromatography (HPLC) with the electrochemical detection (13).
Experimental procedure
Simultaneously to the microdialysis haloperidol-induced, catatonia was induced with haloperidol (1 mg/Kg) and assessed at 30 min intervals until 120 min and at the end of 240 min by means of standard bar test (14, 15). Haloperidol (1 mg/Kg IP) was chosen so that it could elicit a moderate degree of catalepsy and thus enable the detection of either attenuation or potentiation of phenomenon (16, 17). Catalepsy was assessed in terms of the times for which the rat maintained an imposed position with both front limbs extended and resting on a 10 cm high wooden bar (1.25 cm diameter). The end point of catalepsy was considered to occur when the both front paws were removed from the bar or if the animal moved its head in an exploratory manner. A cut-off time of 300 sec was applied. Between the determinations, animals were returned to their individual home cages.
Scoring method: If the animal maintained the imposed posture for at least 20 sec, it was considered to be cataleptic and given one point. For every additional 20 sec that the cataleptic posture was maintained, one extra point was given. The animals were tested twice at 30 min intervals and only the greater duration of immobility was considered. In the acute study, compound 11b (2, 4 and 8 mg/Kg) and scopolamine (1.0 mg/Kg) were administered only 30 min prior to the haloperidol administration. In the Sub-chronic study, these agents were administered once daily 30 min prior to the haloperidol administration for seven days. Catalepsy was determined 30 min after the haloperidol administration on the 1st and 7th day of the treatment.
Statistical analysis
Catalepsy
For each group, mean ± SEM was considered and the data was analyzed by one way ANOVA followed by Dunnett’s Multiple Comparison test. P < 0.05 was considered to be statistically significant.
Microdialysis
The average concentration of four stable samples before the administration of drugs or vehicle [DMSO] was considered as the basal glutamate-dopamine concentration. Statistical evaluation of the results was performed by means of one-way analysis of variance (ANOVA) and Student-Newman-Keuls multiple range test, considering the following significant differences: p < 0.05.
Results and Discussion
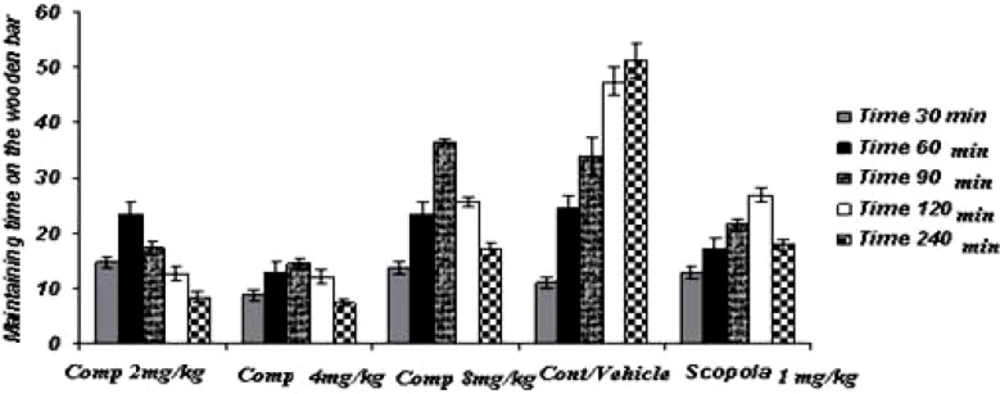
Acute study (Figure 1)
In the acute study, the administration of standard drug (scopolamine) and all doses of the test drug gave cataleptic scores similar to the vehicles-treated groups. However, from 60 min onwards after the haloperidol administration, scopolamine (1.0 mg/Kg) resulted in significantly lower cataleptic scores than the vehicle-treated rat. On the other hand, the median dose and the lower dose of compound 11b resulted in lower cataleptic scores as compared to the vehicle-group, 60 and 90 min after the haloperidol administration, respectively. However, from 120 min onwards until 240 min after the haloperidol administration, the higher dose of compound 11b significantly lowered the cataleptic scores compared to the vehicle group. In fact, 90 min post-haloperidol-induced all doses of compound 11b were actually more protective against catalepsy than even the standard drug scopolamine. Surprisingly, the higher dose of compound 11b was observed to be less effective in catalepsy improvement, comparing to other doses of compound.
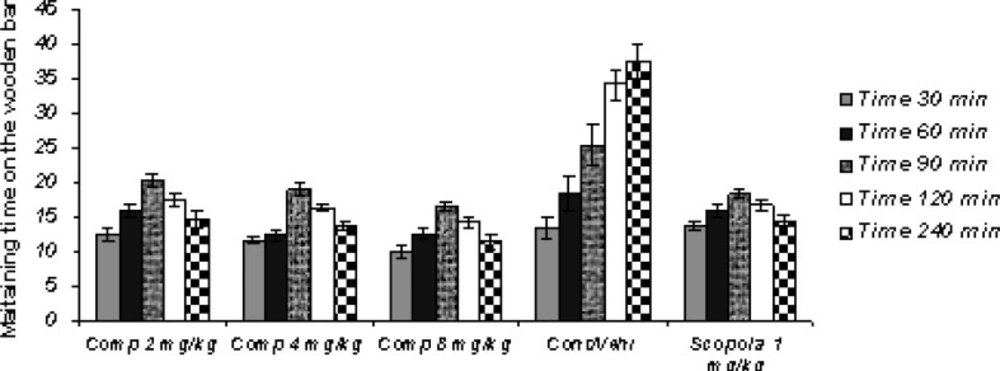
Sub-chronic study (Figure 2)
In the Sub-chronic study, the administration of standard drug and all doses of the test drug 30 min after the last haloperidol dose on the 7th day, gave cataleptic scores similar to the vehicles-treated groups. However, from 60 min onwards after the haloperidol administration, scopolamine (1.0 mg/Kg) and compound 11b (2, 4 and 8 mg/Kg) resulted in significantly lower cataleptic scores than the vehicle-treated rat. The Sub-chronic results of the study showed that the highest dose of compound 11b was as protective as the 1 mg/Kg dose of scopolamine. Our statistical analysis showed that the protective effect of compound 11b against haloperidol-induced-catatonia was both dose-and time-dependent.
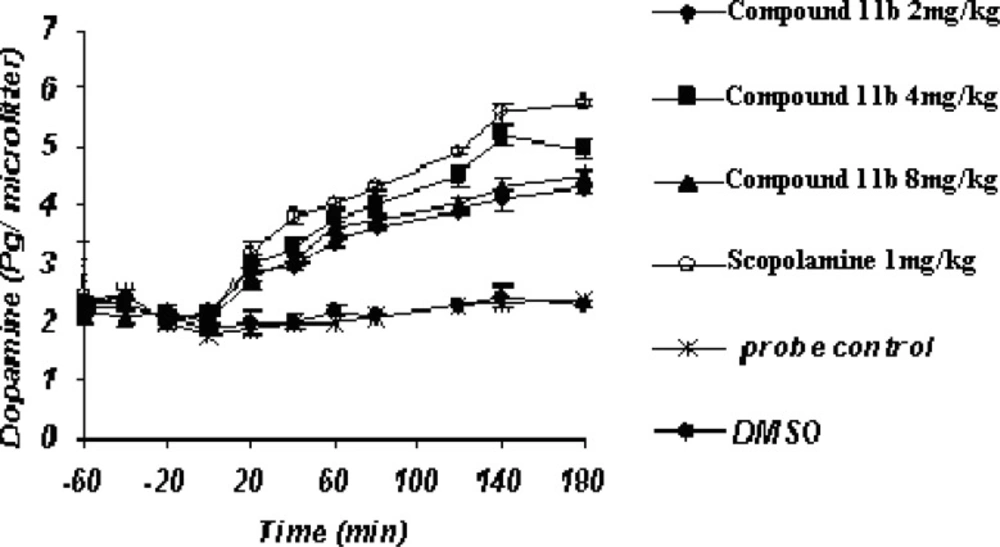
Acute microdialysis study (Figure 3)
The striatal extraneuronal (i.e. microdialysate) concentration of dopamine in rats of all examined groups before the drug-vehicle injection (baseline) was 2.1 ± 0.33 pg/20 μL respectively.
COX-2 inhibitor and their doses as well as scopolamine were observed to modify dopaminergic neurotransmission in the striatum of rats during the observation period. Additionally, the changes were seen to be significant (p < 0.05) for dopamine concentrations on or after 40 min throughout the process until 180 min.
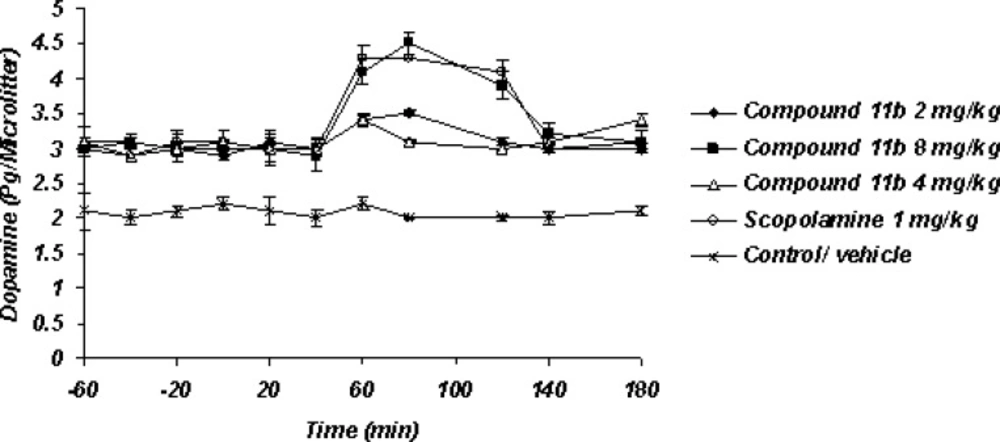
Sub-chronic microdialysis study (Figure 4)
The striatal extraneuronal (i.e. microdialysate) concentration of dopamine in rats of all examined groups were 2.9 ± 0.41 pg/20 μL respectively before the drug-vehicle injection (baseline). This observation shows that sub-chronic administration of selective COX-2 inhibitor compound 11b can elevate the basal level of Striatum dopamine from 2.1 ± 0.33 pg/20 μL to 2.8 ± 0.41 pg/20 μL. The day 7th, results of the microdialysis experiment showed the significant increasing level of striatal dopaminergic neurotransmission with regard to the 7th day basal level after the administration of Selective COX-2 inhibitor but just with its high dose 8 mg/Kg as well as scopolamine 1 mg/Kg within 40-120 min observation period.
This study was intended to explore the more important role of COX-2 in dopaminergic neurotransmission in striatum of rats. Also this study shows that acute and sub-chronic COX-2 selective inhibitor compound 11b administration had an important role in haloperidol-induced catalepsy improvement by enhancing the dopaminergic neurotransmission.
It seems that COX-2 and its metabolites, prostaglandins (PGs), have an important role in neurotransmitter release and PD affiliated rigidity as noted in previous reports )4, 5, 18) suggesting that COX-2 causes increased levels of acetylcholine in the brain via production of PGE2 and increases in expression of cholinergic markers, such as choline acetyl transferase and vesicular acetylcholine transporter protein. It was also noted that prostaglandins have modulatory effects on adrenergic, noradrenergic and glutaminergic transmission, specially PGE2, and prostaglandin synthesis inhibitors induced increases in the blood pressure via increases in the release of the catecholamine; for example using large doses of glucocorticoids in humans may cause insomnia, euphoria and increase the intracranial pressure. Additionally, an in-vitro study evaluated the effects of some NSAIDs on cultured primary rat embryonic neurons from rat embryos mesencephalon also containing glial cells, an experimental preparation that reflected the cellular composition of the brain, useful in the study of neuroinflammation. Incubation with aspirin, paracetamol or ibuprofen protected dopaminergic neurons against the glutamate toxicity. The study considered as indices the reduction of the decrease in dopaminergic uptake caused by glutamate, and the attenuation of the tyrosine hydroxylase positive cells loss. Among the tested NSAIDs, ibuprofen was the most effective one and surprisingly increased the number of dopaminergic cells in the basal condition, most likely protecting them from the excitotoxicity associated with culture medium change. In the present research, we showed in subchronic study that only high doses of Compound 11b were able to modify the Dopamine levels. It has been shown that normally, COX-2 is expressed in low levels in nigral dopaminergic neurons, but it becomes up-regulated in both patients and experimental PD models (19). These suggest that different levels of COX-2 in normal and suprachiasmatic nuclei-lesioned (SCN-lesioned) rats may explain the aforementioned observations.
Conclusion
Due to a lot of difficulties such as lack of technical and financial supports, we could not investigate the effect of Compound 11b on other related neurotransmitter systems such as cholinergic-glutaminergic neurotransmissions and these should be further investigated in the future experiments.