Introduction
It is projected that incidence of diabetes is on rise. Present number of diabetics worldwide is 150 million and this is likely to increase to 300 million or more by the year 2025. Reasons for this rise include increase in sedentary lifestyle, consumption of energy rich diet, obesity, higher life span, etc. (1). Though biguanides and sulfonylureas are valuable in treatment of diabetes mellitus, their use is restricted by their limited action, pharmacokinetic properties, secondary failure rates and accompanying side effects (2). Moreover, these therapies only partially compensate for metabolic derangements seen in diabetics and do not necessarily correct the fundamental biochemical lesion (3). Nature has been a source of medicinal treatments for thousands of years, and plants-based systems continue to play an essential role in the primary health care of 80% of the world’s underdeveloped and developing countries (4).
In insulin induced dependent diabetes mellitus (IDDM) various agents like interlukin-1 beta, interferon gamma, tumour necrosis factor alpha, alloxan and streptozotocin- could operate by forming free radicals that could attack the mitochondrial genome. (5). The increased production of free radicals in mitochondria
may damage β-cells, which is known to be very sensitive to free radicals (6). Also decrease in oxygen consumption and respiratory ratio were observed by (7). Furthermore, lowering in the activities of pyruvate dehydrogenase and increase in NAD+/NADH ratio were reported in alloxan-induced diabetic rats (8). It has been suggested that the diabetogenicity of streptozotocin is dependent on the inhibition of the activities of citric acid cycle enzymes like isocitrate and α-ketoglutarate dehydrogenase (9).
In the present study we have selected a plant PimpinellatirupatiensisBal. &Subr. (Family Apiaceae; local name, kondakothimera) is a rare and endemic medicinal plant and restricted to the Seshachalam hills of the Eastern Ghats, India (10-12). Dried roots of Pt are administered along with few other ingredients to cure colic and rheumatic ailments in cattle (13). The local Yanadhi tribal community uses the tuberous roots of Pt to cure severe ulcers of stomach, throat and genital organs and also as aphrodisiac (14) and abortifacient agents (15). Fruits are used to cure asthma and are considered as an effective remedy for ‘flatulent colic’ (14).The whole plant of Pt is used to treat cough, stomach, liver problems, asthma, ulcer and tooth ache (16,17). This plant root extract is also used to treat skin disease (18) and is used as an antimicrobial agent (19) it is even given in the treatment of venereal disease and peptic ulcers (20).
There is no scientific basis for antioxidant, antidiabetic activity of Pt. There were no reports on the activity of Pt in diabetic rats with reference to carbohydrate metabolic profiles. The present work was undertaken to evaluate the effects of Pt root extract in the diabetes associated alterations in oxidative enzymes.
Experimental
Chemicals
All the chemicals used in the present study were analar Grade (AR) and obtained from the following significant companies: Sigma (St.Louis, MO, USA), Fischer (Pitrsburg, PA, USA), MErk (Mumbai, India), Ranbaxy (New Delhi, India), Qualigens (Mumbai, India).
Animals
Wistar strain male albino rats, aged 3 months (200-250 g) were used for the present study. The total number of animals used for this study is 30. The rats were maintained on standard pellet diet and provided access to water ad libitum. They were housed in clean, dry polypropylene cages and maintained in a well ventilated animal house with 12 h light-12 h dark cycle. All the experiments were carried out between 8 am to 10 am in order to avoid circadian rhythm induced changes.
Induction of diabetes
Diabetes was induced in healthy male Wistar Albino rats aged about 3 months, with body weights ranging from 200-250 g, by a single intra peritoneal injection of freshly prepared STZ (40 mg/kg b.w) dissolved in ice cold 0.1 M citrate buffer (pH 4.5) after allowing the rats for overnight fasting for 12-15 h as per the method followed by Rakietenet al., (21). 8 hrs after STZ administration the rats were kept for next 24 h on given 15% glucose solution to prevent hypoglycemia, as STZ is capable of producing fatal hypoglycemia due to destruction of β cells which in turn results in to massive pancreatic insulin release. Diabetes was assessed by determining the fasting blood glucose after 48 h of injection of STZ. The blood glucose levels in STZ rats were increased to markedly higher levels than normal. After a week, when the condition of diabetes was stabilized, rats with marked hyperglycemia (blood glucose level ≥ 250 mg/dL) were selected. Blood was collected from the tail vein.
The protocol of this study was submitted to the Institutional Animal Ethics Committee and approved in its resolution No 09 (iii)/a/CPCSCA/IAEC/07-08/SVU/Zool/KSR-SRR/dated 26/6/08.
Plant material and extraction
Tuberous roots of Pimpinellatirupatiensis(Pt) were collected from Shesachalam hills, (Chittoor district, Andhra Pradesh, India) during the raining season and identified by the Taxonomist of the Herbarium, Department of Botany, S.V.University, Tirupathi. Voucher specimen (1533) was deposited in S.V.University, Tirupati, Andra Pradesh, India. These roots were air dried and powdered .The powder was stored in airtight containers and was used for the extraction. To 500 g of root powder, 1500 mL of ethyl alcohol was added the clear filtrate was evaporated to dryness under vaccum using the rotavapor at 35-40°C and further dried by freeze drying.
Experimental design
The rats were divided into 5 groups, six rats in each group and treated as follows:
Group I: Normal control (NC), Group II: diabetic control (DC), Group III: (D+Ea.e): Diabetic animals were treated orally with 750 mg/kg/day of Pt ethyl alcohol extract for 30 days, Group IV:(N+Ea.e): Normal animals were treated orally with 750 mg/kg b.w/day of pt ethyl alcohol extract for 30 days, Group V: (D+Glb): Diabetic animals were treated with 20 mg/kg b.w/day of Glibenclamide for 30 days.
Analytical procedures
After completion of 30 days treatment the animals were sacrificed by cervical dislocation and the kidney tissue was excised at 4oC. The tissue was washed with ice-cold saline, immersed in liquid nitrogen and immediately stored in deep freezer at -80°C for further biochemical analysis.
Estimation of Succinate dehydrogenase activity (Succinate acceptor oxidoreductase–E.C: 1.3.99.1); 10% (w/v) homogenates of the kidney tissues were prepared in ice cold 0.25 M sucrose solution and centrifuged at 1000 g for 15 min at 4°C. The supernatant fraction was used for enzyme assay. The reaction mixture in a final volume of 2 mL contained 40 μ moles of sodium succinate, and 100 μmol of phosphate buffer (pH 7.0) and 4 μmol of INT. The reaction was initiated by adding 0.2 mL of homogenate containing 20 mg of tissue as an enzyme source. The incubation was carried out for 15 min at 37°C and the reaction was stopped by the addition of 5 mL of glacial acetic acid. Zero time controls (ZTC) were maintained by addition of 5 mL of glacial acetic acid prior to the addition of the enzyme source to the incubation mixture. The formazan formed was extracted over night into 5 mL of toluene at 5°C. The color developed was measured at 495 nm in a Spectrophotometer against the toluene blank. The enzyme activity was expressed in μ moles of formazan formed/mg protein/h (22, 23)
Estimation of Malate dehydrogenase (MDH) (L-malate NAD+oxidoreductase–E.C: 1.1.1.37); 10% (w/v) homogenates of the Kidney tissues were prepared in ice cold 0.25 M sucrose solution and centrifuged at 1000g for 15 min at 4°C. The supernatant fraction was used for enzyme assay. The total volume 2 mL of reaction mixture contained 100 μmol of phosphate buffer (pH 7.0) 40 μmol of sodium malate, 0.1 μmol of NAD and 4 μmol of INT. The reaction was initiated by the addition of 0.2 mL of homogenate containing 20 mg of tissue as an enzyme source. The incubation was carried out at 37°C for 30 min and the reaction was arrested by adding 5 mL of glacial acetic acid. The rest of the procedure was same as described earlier for LDH. The activity was expressed in μmol of formazan formed / mg protein / h (22, 23).
Estimation of Glutamate dehydrogenase (GDH-L-Glutamate; NAD oxidoreductase – EC: 1.4.1.3); 5% (W/V) of tissue homogenates were prepared in ice cold sucrose (0.25M) solution and the contents were centrifuged at 1000 g for 15 min at 4°C. The supernatant part was used as an enzyme source. The reaction mixture in a total volume of 2 mL contained 100 μmol of phosphate buffer (pH 7.4), 40 μmol of sodium glutamate, 0.1 μmol of NAD, 2 μmol of INT and 0.2 mL containing 10 mg of tissue as an enzyme source. The reaction mixture was incubated at 37°C for 30 min. The reaction was arrested by the addition of 5 ml glacial acetic acid and the formazan formed was extracted into 5 mL of toluene. The intensity of the color was read at 495 nm against the toluene blank. The enzyme activity was expressed as μmol of formazan formed/mg protein/h (24).
Estimation of Isocitrate dehydrogenase (ICDH) (Isocitrate: NADP+ oxidoreductase E.C:1.1.1.42); 10% homogenates of kidney tissues was prepared in 0.25 M ice cold sucrose solution and centrifuged at 1000 g for 15 min at 4°C. The supernatant was used for the enzyme assay. The reaction mixture in a final volume of 2.0 mL contained 40 μmol of DL-isocitrate 100 μmol of magnesium chloride, 100 μmol of sodium phosphate buffer (pH 7.4), 4 μmol of INT (2-P-iodophenyl 3-P-nitrophenyl 5-phenyl tetrazolium chloride), 0.2 μmol of ADP and 0.2 μmol of NADP (for NADP+-ICDH).
The reaction was initiated by the addition of 0.2 mL supernatant containing 20 mg of the enzyme source and the contents were incubated at 37oC for 30 min. After incubation, the reaction was stopped by adding 5.0 mL of glacial acetic acid and the formazan formed was extracted overnight at 5oC into 5.0 mL of toluene. The colour was measured at 495 nm in a spectrophotometer against toluene blank. The enzyme activity was expressed as μmol of formazan formed/mg protein/h (25, 26).
Estimation of Lactate dehydrogenase (LDH) (L-lactate: NAD+ Oxidoreductase–E.C: 1.1.1.27); 10% (w/v) homogenates of the kidney tissues were prepared in ice cold 0.25 M sucrose solution and centrifuged at 1000 g for 15 min at 4oC. The supernatant fraction was used for enzyme assay. The reaction mixture in a final volume of 2 mL contained 40 μmol of sodium lactate, 100 μmol of phosphate buffer (pH 7.4), 0.1 μmol of NAD and 4 μmol of INT. The reaction was initiated by the addition of 0.2 mL of homogenate containing 20 mg of tissue as an enzyme source and incubated for 30 min at 37oC and the reaction was stopped by the addition of 5 mL of glacial acetic acid. Zero time controls (ZTC) were maintained by addition of 5 mL of glacial acetic acid prior to the addition of the enzyme source to the incubation mixture. The formazan formed was extracted over night into 5 mL of toluene at 50C. The color developed was measured at 495 nm in a Spectrophotometer against the toluene blank. The enzyme activity was expressed in μmoles of formazan formed/mg protein/h (22, 23).
Statistical analysis
The data has been analyzed by using SPSS (Version 13.5; SPSS Inc., Chicago, IL, USA) and M.S.Office, Exel Software for the significance of the main effects (factors), and treatments along with their interactions. The data has been compared using one-way ANOVA with Duncon’s multiple range test (DMRT) and differences were considered significant at p < 0.01.
Results
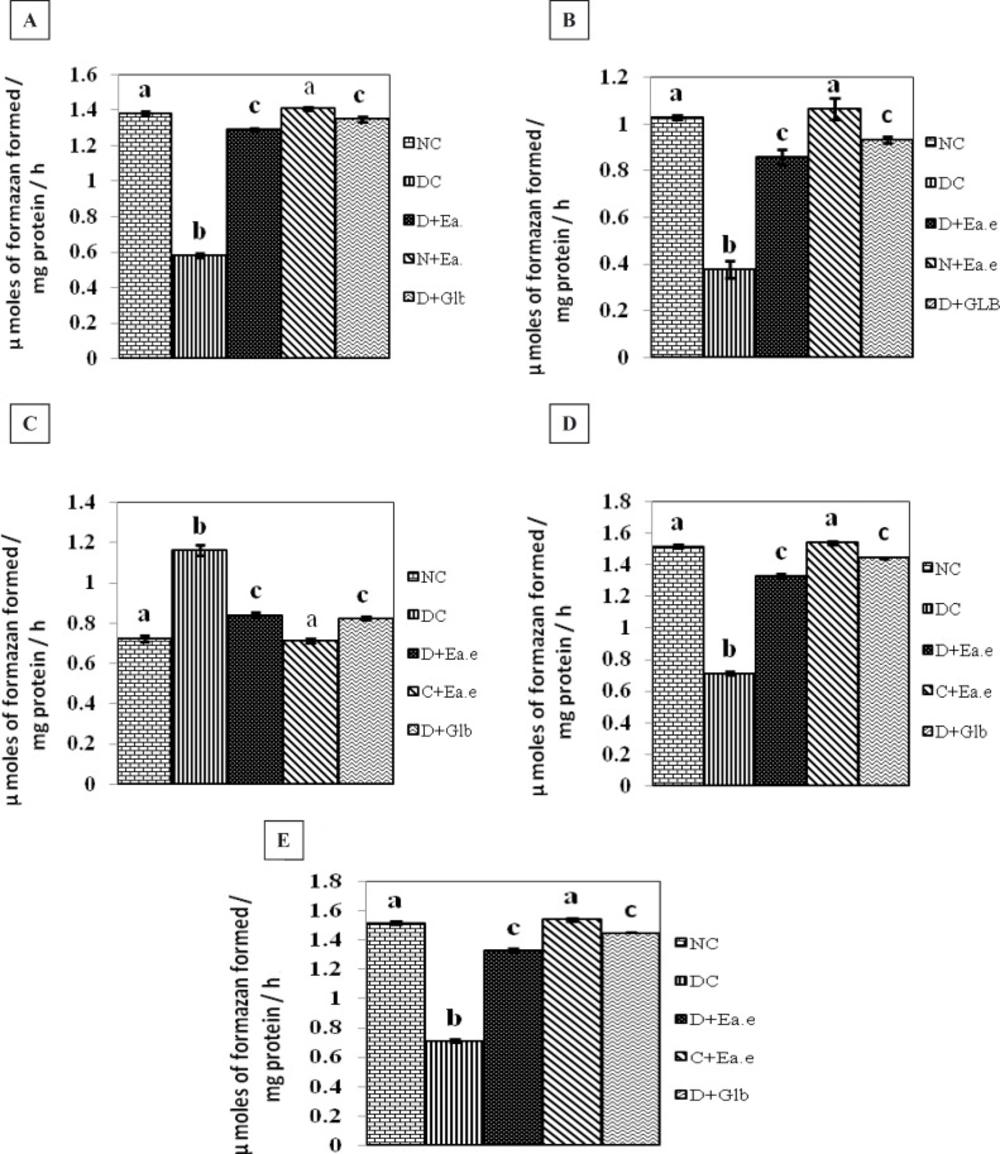
The effect of Pt supplementation on the activities of Succinate dehydrogenase (SDH), Malate dehydrogenase (MDH), Lactate dehydrogenase (LDH), Isocitrate dehydrogenase (ICDH), Glutamate dehydrogenase (GDH), in the kidney of control and experimental groups of rats are shown in Figure 1 (A-E). There were no significant changes in the activity of these parameters in control rats treated with Pt alone. However, the activities of SDH, ICDH, GDH, MDH were decreased significantly (p < 0.01) and the activity of LDH was significantly (p < 0.01) increased in the kidney of STZ-induced diabetic rats. Treatment with Pt to diabetic groups of rats, similar to glibenclamide (GLB), the altered activities of these enzymes were regulated significantly (p < 0.01) to near normalcy in kidney.
Activities of SDH, MDH, LDH, ICDH and GDH respectively in the kidney of Normal control rats (NC), Diabetic control rats (DC), Diabetic rats treated with Pt Ethyl alcohol extract (D+Ea.e), Normal rats treated with Pt Ethyl alcohol extract (N+Ea.e), Diabetic rats treated with Glibenclamide (D+Glb). Each vertical bar represents the mean ± SE (n = 6). Top of the vertical bars having the same letter do not differ significantly at p < 0.01
Discussion
In the present study, the observed decrease in the activities of mitochondrial enzymes in the kidney of the diabetic rats were significantly enhanced upon Pt treatment (Figure 1 (A-E)). In insulin dependent diabetes mellitus (IDDM) various agents like interleukin-1 beta, interferon gamma, tumor necrosis factor alpha, alloxan and streptozotocin - could operate by forming free radicals that could attack the mitochondrial genome (5). The increased production of free radicals in mitochondria may damage β-cells, which is known to be very sensitive to free radicals (6). Also, a decrease in oxygen consumption and respiratory ratio were observed in the mitochondria of diabetic rats (27). A similar decrease in the activities of citric acid cycle enzymes were also observed by Seneret al., (7). Furthermore, lowering in the activities of malate dehydrogenase and increase in NAD+/NADH ratio were reported by Obrosovaet al., (8) in alloxan-induced diabetic rats. It has been suggested that the diabetogenicity of streptozotocin is dependent on the inhibition of the activities of citric acid cycle enzymes like isocitrate and α-ketoglutarate dehydrogenase (9). In diabetes mellitus, abnormalities of mitochondrial enzymes may impair the metabolism of glucose. As the rate of glucose oxidation normalizes insulin secretion and subsequent release of β-cells, a defective insulin response to glucose stimulation may be due to respiratory chain deficiency in the pancreas of IDDM. This supposes that random partitioning of mitochondria during development might have resulted in the accumulation of mutated mitochondrial DNA-containing fragments in particular tissues including pancreas. The rearrangements produce potentially antigenic chimeric proteins. The reduction in the functioning of mitochondrial enzymes may lead to a defect in the mitochondrial energy production which would impair protein synthesis and energy production in β-cell (28). Restoration of the activities of mitochondrial enzymes on Pt therapy suggests that it may be beneficial in enhancing protein synthesis and energy production.
The decrease in GDH activity is attributed to its inhibition by elevated ammonia levels (product-inhibitor), which diminish the catalytic efficiency of the enzyme molecule (29). Reddy and Rao, (30) demonstrated that increased ammonia and lactate levels inhibit GDH activity. The increased LDH also reported in the present study, consonance with that lactate inhibits the GDH activity. The decrease in the activity of GDH suggests that regulation of ammonia toxicity in the kidney by the processes of deamination and amination is affected during the diabetic state. This was also reported in brain of diabetic rats by Telushkinet al., (31). The decrease in activities of GDH in the brain of rats with enzyme dysfunction was due to activation of lipidperoxidation (31), which attests to serious disturbances in energy metabolism and contributes to the impairment of glutamate utilization in the brain and progression of glutamate induced toxicity. The increased activity might be due to the decreased oxidative stress by Pt and increase the mitochondrial enzymes. Pt has the capacity to increase the activity of GDH in diabetic rats. There are many reports on inhibition of GDH activity by medicinal plants in diabetic rats. Trigonella treatment for 21 days to diabetic rats normalization of mitochondrial enzymes in diabetic rats (32).
In the current study MDH activity was decreased in diabetic rat kidney tissue. The decrease in specific activity of MDH as a consequence of diabetes suggests decreased utilization of malate. The decrease in the activity levels of dehyddrogenases is in consistent with the decreased conformation (33). An increase in proteolytic activity during diabetes may also be responsible for the decreased MDH activity. MDH activity was decreased in the tissues of diabetic animals in several studies (34, 35). The increased production of free radicals in mitochondrial cells in the tissue, also a decrease in oxygen consumption respiratory ratio were observed in mitochondria of diabetic rats. (27). Lowering in the activity of MDH and increase in NAD+/NADH were reported by Obrosovaet al., (36). It has been suggested that the diabetogenecity of STZ is dependent on the inhibition of the activities of citric acid enzymes like MDH, α-ketoglutarate dehydrogenase (9). Diabetes decreased the expression of genes involved in carbohydrate and energy metabolism through effects on known pathways such as glycolysis, TCA cycle and oxidative phosphorylation. In diabetic rats with Pt treatment MDH levels were elevated. This may be due to decreased oxidative stress and increased activities of mitochondrial enzymes. Pt has the capacity to increase the activities of mitochondrial enzymes, this may due to the compounds which are present in Pt has the capacity to decrease the oxidative stress and increase these mitochondrial enzymes activities. There are many reports on normalization of MDH activity with medicinal plants treatment in diabetic rats. The observed decrease in the activities of mitochondrial enzymes in liver and kidney of the diabetic rats were significantly enhanced upon molybdate therapy (35). C-peptide rectified the mitochondrial defects and corrects many of the maladies associated with diabetes (37).
SDH activity was reported to be inhibited in tissues of diabetic animals in several studies (6, 35, 38-40). The decreased activity of SDH in diabetic condition affecting succinate-fumarate conversion indicates the depressed oxidative metabolism at the level of mitochondria. A similar decrease in the activities of citric acid cycle enzymes were also observed by Seneret al., (7). Hyperglycemia results in decreased activities of citric acid enzymes and pentose phosphate pathways enzymes. As the phosphorylated glucose enters into the pathways like glucogenesis and glycoprotein synthesis (39, 41, 42)
The deleterious effects of oxidative stress on mitochondrial respiration, ATP synthesis and membrane properties are mainly connected with extensive peroxidation of membranous polyunsaturated phospholipids, the integrity of which is important for functioning of mitochondrial respiratory chain. The damage of these lipids surrounding membrane bound enzymes alters the function of these enzymes (43), primarily those of mitochondrial dehydrogenases (44, 45). Long term reactive oxygen species exposure to oxidative stress resulted in oxidative damage of mitochondrial proteins that caused disturbances in mitochondrial energy production.
The decrease in SDH activity due to the STZ induced oxidative stress condition indicates reduction in the conversion of succinate to fumarate resulting in decreased in oxidative metabolism. During stress condition diversion of phosphoenolpyruvate leads to increased formation of fumarate resulting in product inhibition of SDH (46). The decrease in the activities of SDH in tissues of diabetic rats can be associated with enzyme dysfunction due to activation of lipid peroxidation. This may be due to excess production of free radicals to counter these toxic effects. In diabetic rats treated with Pt SDH activity was increased when compared to control diabetic rats. This elevation may be due to the compounds present in Pt. There are many reports on the reduction of oxidative stress by plants (35) and also plants has the capacity in normalizing the levels of lipid peroxidation. Hence by normalizing the levels of lipids the mitochondrial enzymes may become to normal level more or less in diabetic rats treated with Pt treatment. Increase in SDH activity in Pt treated rats indicates better utilization of energy yielding intermediates by TCA cycle. Same results were seen in UDCA (Ursodeoxycholic acid) treated diabetic and alcohol treated rats. This acid ameliorates the oxidative phosphorylation and normalizing mitochondrial enzymes (47).
Lactate dehydrogenase (LDH) is a terminal glycolytic enzyme that plays an indispensable role in the interconversion of pyruvate to lactate to yield energy under anaerobic conditions (48) and the reaction occurs in both cytosolic and mitochondrial compartments (47). LDH activity is found to be altered by insulin, glucose, NADH, as well as increases in mitochondrial membrane potential, cytosolic free ATP and cytosolic free Ca2+ (50). The decreased activity of LDH in tissues could be important to ensure that a high proportion of both pyruvate and NADH, supplied by glycolysis, is subsequently oxidized by mitochondria. This excessive pyruvate is converted to lactate for which LDH is needed and there fore the activity of LDH may be increased due to less insulin availability in diabetes (51, 53). Increased LDH activity in diabetes has been reported by Ramachandranet al., (53). The results of the present study indicates the kidney LDH activity in rats with diabetes were significantly higher when compared to control. (54). Alloxan induced diabetes caused lipid peroxide mediate tissue damage in the liver, kidney, and heart (55). These changes can alter the properties and functions of the cell, resulting in either increased synthesis of some enzymes. Goldberg et al., (56) indicated that LDH levels were higher in patients with diabetes, than those in normal subjects. Indeed, elevated LDH levels observed in the experimental diabetic animals are associated with impaired glucose-stimulated insulin secretion (57).Thus, increased activity of LDH interferes with normal glucose metabolism and insulin secretion in the β-cells of pancreas and it may therefore be directly responsible for insulin secretory defects in diabetes. However, treatment with Pt to diabetic rats reverted the LDH activity to near normalcy. Similarly treatment with resveratrol to diabetic rats decreased the activity of LDH (58) most probably by regulating the proportion of pyruvate and NADH thereby promoting the mitochondrial oxidation of (pyruvate) glucose. The protective effects due to treatment with Pt strongly indicate the possibility of the extract being able to prevent any leakages of marker enzymes. There are some reports on reversal of LDH in diabetic rats with treatment with Murray koenigii, Ocimum sanctum (59). Stefen and Irwin (60) reported that vandate stimulates the oxidation of NADH, and hence the reduced activity of LDH in vanadyl-treated diabetic rats.
The Isocitrate dehydrogenase (ICDH) catalyze oxidative decarboxylation of isocitrate to α-ketoglutarate and require either NAD+ or NADP+ producing NADH and NADPH, respectively (61). NADPH is an essential reducing equilent for the regeneration of reduced Glutathione (GSH) by Glutathione reductase and for the activity of the NADPH-dependent thioredoxin system (62, 63 ) both are important in the protection of cells from oxidative damage. Therefore, ICDH may play an antioxidant role during oxidative stress. ICDH is involved in the supply of NADPH needed for GSH production against mitochondrial and cytosolic oxidative damage (64, 65). Hence, the damage of ICDH may result in the perturbation of the balance between oxidants and antioxidants and subsequently lead to a pro-oxidant condition. We determined the activity of isocitrate dehydrogenase (ICDH) in STZ-induced diabetic rats, ICDH activity in the diabetic group was significantly lower than that in the control group. Similar results are reported by Kilet al., (66) they reported that mitochondrial ICDH activity was lower in diabetic group than control group.The activity of ICDH can be inhibited by glycation of ICDH. Reactive oxygen species contribute to the inactivation of ICDH by glycation. After treating with Pt the activity of ICDH was normalized, this could be due to the antioxidant activity of Pt.