Introduction
Oxidative stress, an excessive production of reactive oxygen species (ROS) above the body’s antioxidant capacity, has been implicated in the development of many pathophysiological conditions including diabetes, hypertension, atherosclerosis, cancer and the process of aging (1, 2). Various studies have shown that diabetes mellitus is associated with oxidative stress, leading to an increased production of reactive oxygen species (ROS), including superoxide radical (O2•-), hydrogen peroxide (H2O2), and hydroxyl radical (OH•) or reduction of antioxidant defense system (3, 4). The formation of ROS is prevented by an antioxidant system that includs non-enzymatic antioxidants (ascorbic acid, glutathione, tocopherols), enzymes regenerating the reduced forms of antioxidants, and ROS–scavenging enzymes such as superoxide dismutase (SOD), glutathione peroxides (GPX) and catalase (CAT) (5, 6). It is well established that the risk of cardiovascular disease due to atherosclerosis enhance with increasing concentration of total cholesterol and augmented levels of triglycerides in the plasma (7, 8). Patients with type 2 diabetes have a twofold to fourfold excess risk of coronary artery disease as compared to non diabetic patients and many of the primary risk factors for coronary artery disease frequently coexist in these patients (9, 10).
Since the synthetic drugs have undesirable side effects or contraindications, the World Health Organization (WHO) has recommended the evaluation of traditional plants treatments for diabetes (11). Medicinal plants are widely used worldwide to address a variety of health problems. About 25 to 50% of current pharmaceuticals are derived from plants (19, 20). Plants are rich in a wide variety of phytochemical metabolites which are divided into two groups: Primary and Secondary metabolites. Primary metabolites consist of common sugars, amino acids, proteins and chlorophylls, while Secondary metabolites include glycosides, alkaloids, saponins, phenolic compounds, terpenes steroids and anthraquinones (21).
Garlic is known to be effective in decreasing plasma cholesterol and can inhibit LDL oxidation (12). Garlic bulbs active principle agent is allicin, a sulfur-containing compound that with its breakdown products gives to garlic its characteristic odor (13). Also, S-allyl cysteine sulfoxide which is a major antioxidant component of garlic extract has scavenging free radicals property and reduce lipid peroxidation (14). Analysis of Persian shallot extracts has confirmed the presence of flavones and polyphenolic derivatives, suggesting that it also may have antioxidant properties (15, 16). The antioxidant properties of sage extracts is due to its constituents, mainly phenolic compounds such as carnosic, rosmarinic, caffeic and salvianolic acids (17, 18).
In this study we have examined the antioxidant and hypolipidemic effects of methanolic extracts of Allium sativum, Allium ascalonicum and Salvia officinalis in Alloxan induced diabetic rats.
Experimental
Chemicals and reagents
SOD and GPX kits were purchased from Randox (Antrim, UK). The kits for determination of triglyceride (TG), total cholesterol (TC), high-density lipoproteins (HDL) were from ChemEnzyme (Tehran, Iran), Hemoglobin Reagent Set from ZiestChem (Tehran, Iran). All other chemicals and solvents were of the highest commercial grade from Merck (KGaA, Germany) or from Sigma (St. Louis, MO, USA).
Preparation of methanolic extracts: ASE, AAE and SOE
Plants materials used in this study consisted of the bulbs of Allium sativum L. and Allium ascalonicum L. and the leaves of Salvia officinalis L. All plants materials were obtained from Tehran province of Iran. They were authenticated by Professor Ahmad Qahraman and voucher specimen as follows: Allium satium L., 35842, Allium ascalonicum L., 35351 and Salvia officinalis L., 37221 and were deposited at the herbarium of University of Tehran, Tehran, Iran. About 200gr of dried and ground bulbs of garlic and Persian shallot and leaves of sage were extracted with 300 ml methanol (80%) in a Soxhlet apparatus for 72 h. After extraction, the solvent was filtered and then evaporated by Rotavapor. The percentage yields based on the dried starting materials were 20% for garlic, 17% for Persian shallot and 23% for sage. The powders were stored in the dark at 4°C until being used.
Preparation of Alloxan-induced diabetic Wistar rats
Male Wistar rats (Rattus norvegicus allivias), weighing 200-250 g were used in this study (Pasteur Institute, Tehran, Iran). Animals were housed six per standard rat cage, in a room with a 12:12 h light/dark cycle and controlled temperature (22 ± 1°C). There were six rats per group in each experiment. The procedures were performed in accordance with institutional guidelines for animal care and use. Diabetes was induced in overnight fasted rats by subcutaneous injection of Alloxan monohydrate (100 mg Kg-1 , Sigma, St. Louis, MO, USA), dissolved in citrate buffer (pH = 4.5), according to a previously described method (22, 23).
Experimental design
The rats were divided into the six groups, each with six animals. Group I (NC): Normal rats treated with vehicle alone; Group II (DC): Diabetic rats treated with vehicle alone; Group III (ASE+D): Diabetic rats treated with ASE at the dose of 500 mg kg−1 BW; Group IV (AAE+D) : Diabetic rats treated with AAE at the dose of 500 mg kg−1 BW; Group V (SOE+D): Diabetic rats treated with SOE at the dose of 250 mg Kg-1 BW; Group VI (Met+D): Diabetic rats treated with metformin, 100 mg Kg-1 BW.
At the end of 21 days of treatment, rats were anesthetized by ether, their blood was collected in both ordinary and EDTA coated tubes for the estimation of plasma lipids levels and superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase (CAT) activities. Metformin was used as the reference drug.
Estimation of antioxidant enzymes activities
The blood of 21 days treated rats were collected in EDTA coated tubes. The activities of SOD (EC: 1.15.1.1) and GPX (EC: 1.11.1.9) were measured using commercial kits. CAT (EC: 1.11.1.6) activity was measured by method of Aebi (24). Activities were expressed as K or U/gHb. The total hemoglobin of samples was measured by a hemoglobin Reagent kit.
Estimation of blood lipids
After 21 days of treatment, rats were anesthetized using ether, their blood was collected from heart in ordinary tubes, was allowed to clot, and then, the clotted blood was centrifuged at 2500 rpm for 5 min. Triglycerides, total cholesterol and high-density lipoproteins were estimated using commercial kits. Low-density lipoproteins and very low-density lipoproteins were calculated by formula (25).
Phytochemical analysis of ASE, AAE and SOE
Phytochemical analysis was carried out on the plants crude extracts. The total phenolic content for each extract was analyzed with the Folin-Ciocalteau method and was expressed as gallic acid equivalents (GAE) in milligrams per gram dry material (26).The method of Allen’s commercial organic analysis was used for flavonoids determination (27). Alkaloids were determined by method of Henry (28). Glycosides were measured using the method of Analytical Committee of Royal Society of Chemistry. The method of Brunner (29) was used for Saponins determination and the method of strumeyer and malin (30) was used for Tannins.
Statistical analysis
All data are presented as means ± S.D. for six rats in each group. Comparisons between groups and between time points were made by one-way analysis of variance (ANOVA) followed by Duncan’s test to analyze the difference. Differences were considered significant when p-values were less than 0.05. All statistical analyses were performed using SPSS (SPSS Inc, Chicago, USA).
Results and Discussion
Effects of ASE, AAE and SOE on antioxidant enzymes activities
The effects of ASE, AAE and SOE are demonstrated in Table 1. As compared with DC group, in ASE+D group, SOD, GPX and CAT activities significantly increased (60%, p < 0.0001), (27%, p < 0.001) and ( 63%, p < 0.043) respectively. In AAE+D group, significant increases were observed in SOD, GPX and CAT activities (65%, p < 0.0001), (43%, p < 0.0001) and (55%, p < 0.043) respectively. In SOE+D group significant raise in SOD (33%, p < 0.0001) and GPX (24%, p < 0.001) activities was observed. Metformin enhanced only slightly the activity of SOD (8% p < 0.043) in comparison with DC group.
| Groups | Dose (mg/Kg bw) | Blood antioxidant enzymes | ||
|---|---|---|---|---|
| SOD (U/g Hb) | GPX (U/g Hb) | CAT (K/g Hb) | ||
| NC | - | 2943 ± 104.4 a | 51.31±4.2a | 1.57 ± 0.6a |
| DC | - | 1945.8 ± 21.6 | 33.11 ± 3.74 | 0.541 ± 0.011 |
| ASE+D | 500 | 3125.4 ± 54.2 a | 42.2 ± 5.21b | 0.882 ± 0.12 c |
| AAE+D | 500 | 3214.3 ± 83.2 a | 47.5 ± 4.2 a | 0.839 ± 0.09 c |
| SOE+D | 250 | 2591 ± 9.13 a | 41.33 ± 2.08 b | 0.598 ± 0.04 e |
| Met+D | 100 | 2118.1 ± 29.7 c | 37.31 ± 5.51 e | 0.551 ± 0.07 e |
Effects of ASE, AAE and SOE on blood lipids levels
Table 2 shows the effects of ASE, AAE and SOE on blood lipids levels in Alloxan induced diabetic rats. In ASE+D, AAE+D, SOE+D and Met+D groups, TG significantly reduced (21%, p < 0.001), (35%, p < 0.0001), (40%, p < 0.0001) and (12%, p < 0.041) respectively, as compared with DC group. In comparison with diabetic control group, ASE, AAE and SOE significantly reduce TC (34%, p < 0.0001), (22%, p < 0.001) and (24%, p < 0.001) respectively. LDL reduced mainly in AAE+D group (28%, p < 0.001), SOE+D group (30%, p < 0.001) and ASE+D group (16%, p < 0.041). In AAE+D and SOE+D groups, VLDL reduced significantly (24%, p < 0.001) and (22%, p< 0.001) respectively. In comparison with DC group, HDL shows no difference in plants extracts treated groups (p > 0.156).
| Groups | Dose (mg/Kg bw) | Serum lipids (mg/dL) | ||||
|---|---|---|---|---|---|---|
| TG | TC | HDL | LDL | VLDL | ||
| NC | - | 85.2 ± 4.8 a | 79.5 ± 4.8 a | 39.2 ± 5.1 a | 21.2 ± 5.6 a | 15.3 ± 2.4 a |
| DC | - | 147.6 ± 7.1 | 118.3 ± 7.8 | 28.6 ± 5.6 | 46.9 ± 11.4 | 26.3 ± 2.1 |
| ASE+D | 500 | 115.4 ± 6.4 b | 77 ± 6.2 a | 31.4 ± 3.7 e | 39.3 ± 7.2 c | 23.61 ± 2.7 e |
| AAE+D | 500 | 95.3 ± 4.6 a | 91.3 ± 4.7 b | 29.5 ± 5.4 e | 33.6 ± 3.8 b | 19.8 ± 1.32 b |
| SOE+D | 250 | 87.33 ± 3.7 a | 89.33 ± 5 b | 29.6 ± 2.08 e | 32.67 ± 3.51 b | 20.33 ± 1.54 b |
| Met+D | 100 | 129.4 ± 5.3 c | 106.7 ± 7.7 e | 27.1 ± 6.2 e | 42.5 ± 8.9 e | 24.6 ± 1.3 e |
Phytochemical analysis of ASE, AAE and SOE
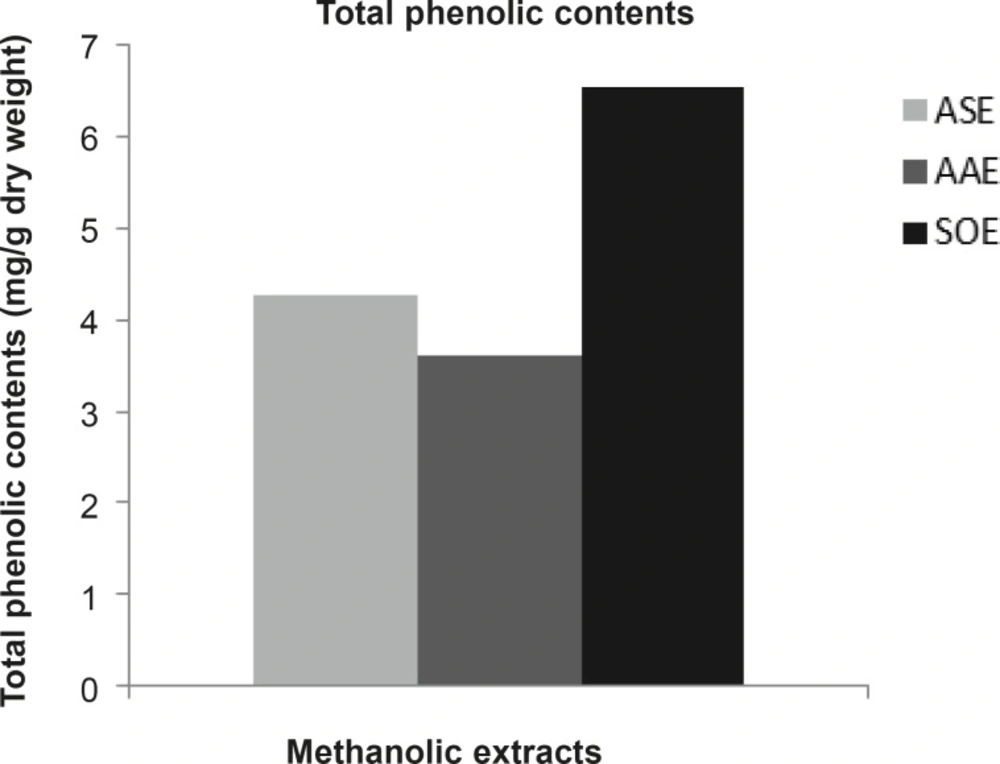
The total phenolic contents of ASE, AAE and SOE were 4.273, 3.621 and 6.548 mg GAE/g dry weight (DW) respectively (Figure 1). The results of phytochemical analyses of methanolic extracts of garlic and Persian shallot showed appreciable amount of glycosides, alkaloids and saponins and lesser amounts of tannins and flavonoids (Table 3). Phytochemical analyses of methanolic extracts of sage showed considerable amounts of glycosides, flavonoids and saponins and trace amounts of tannins and alkaloids (Table 3).
| Phytochemical | Garlic (%) + SD | Shallot (%)+ SD | sage (%) + SD |
|---|---|---|---|
| Flavonoids | 0.049 ± 0.001 | 0.051 ± 0.001 | 1.014 ± 0.071 |
| Alkaloids | 3.490 ± 0.014 | 3.410 ± 0.013 | 0.054 ± 0.001 |
| Saponins | 0.812 ± 0.031 | 0.752 ± 0.011 | 2.096 ± 0.011 |
| Tannins | 0.053 ± 0.001 | 0.049 ± 0.031 | 0.812 ± 0.011 |
| Glycosides | 18.023 ± 0.089 | 13.301 ± 0.172 | 23.142 ± 0.136 |
In diabetes mellitus, hyperglycemia can simply inactivate antioxidant enzymes such as SOD, CAT and GPX by glycating these proteins and induces oxidative stress which in turn causes lipid peroxidation (31, 32). Decreased antioxidant enzymes levels and enhanced lipids peroxidation have been well documented in Alloxan-induced diabetes (33-36). In the enzymatic antioxidant defense system, SOD is one of the important enzymes and scavenges the superoxide radicals by converting them to H2O2 and molecular oxygen. The observed decrease in SOD activity in diabetic control rats could result from inactivation by H2O2 or by glycosylation of the enzyme, which have been reported to occur in diabetes. CAT and GPX are involved in the elimination of H2O2 (37, 38).
Based on the findings of the present study, oral administration of ASE, AAE and SOE increased the antioxidant enzymes levels in red blood cells of Alloxan-diabetic rats. ASE and AAE show noticeable increases in SOD, GPX and CAT activities although SOE have milder effects only on SOD and GPX activities. In conclusion, Decreased levels of SOD and CAT in the diabetic state may be due to inactivation caused by reactive oxygen species. In treatment groups the increased CAT activity could be due to higher production of H2O2. It is possible that CAT activity, which in turn would protect SOD inactivation by H2O2, and would cause an increase in SOD activity. Increase in SOD activity would protect GPX and CAT against inactivation by superoxide anion (39). Meformin, used as a reference drug, also show slight increase in antioxidant enzymes activities which is due to its hypoglycemic effects.
In accordance with phytochemical screening results of ASE, AAE and SOE, flavonoids, glycosides and phenolic compounds found are suggestive of their antioxidant properties.
In type 2 diabetes, dyslipidemia is characterized through increased levels of serum triglycerides (TG) and reduced levels of serum high density lipoproteins (HDL), while total cholesterol (TC) and low density lipoproteins (LDL) may be either normal or marginally elevated (40). The findings of the [resent study show that in diabetic rats, ASE decreases TG, TC and LDL levels while AAE and SOE decrease TG, TC, LDL and VLDL levels. Metformin decreases s TG levels lightly and shows no effects on other plasma lipids. Saponins found in the plants extracts are suggestive of their antihyperlipidemic properties. It was shown that saponins have hypocholesterolemic activities (41).
In conclusion: The major findings of this study is that, in Alloxan diabetic Wisar rats, Allium sativum and Allium ascalonicum bulbs and Salvia officinalis leaves methanolic extracts offer significant protection against oxidative stress and possess capabilities to decrease serum lipids.