Introduction
Cancer is a major health concern all around the world. Progresses in prevention and treatment of cancer have decreased the health rate, but the number of new diagnoses continues to increase. Therefore, new and more efficient anticancer agents are required to battle different cancer diseases. As a part of our efforts to find new chemotherapeutic agents as potential anticancer agent, we have synthesized a series of indole derivatives. Indole derivatives represent many important classes of therapeutical agents in medicinal chemistry such as anti cancer (1), antioxidant (2), anti rheumatoid arthritis (3) and anti HIV (4, 5).
Some studies revealed that a group of 2-phenyl indole sulfamat are steroid sulfatase inhibitors with anti proliferative activity in breast cancer cells (6).
Some of the sulfur containing 2-phenyl indole analogues show in-vivo antineoplastic and anti estrogenic activity (7, 8).
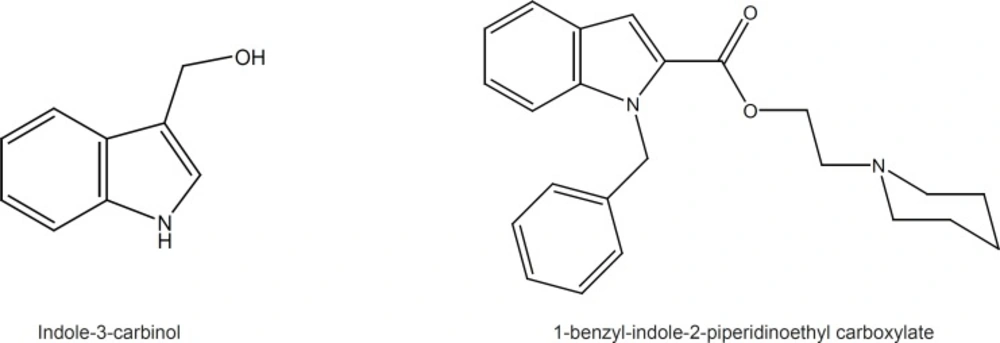
The chemopreventive properties of cruciferus vegetables are attributed to the antitumor activity of indole-3-carbinol (Figure 1) and its metabolic derivatives, which have shown great potential for both prevention and treatment of cancer through numerous mechanisms such as induction of apoptosis and cell cycle arrest, antiestrogenic activity, gene expression modulation and prevention of carcinogen-DNA adduct formation (9,10).
Olgen et al. have viewed indole ring as heterocyclic ATP analogue and discovered a few new indole derivatives with tyrosine kinase inhibitory activity. They have reported that 1-benzyl-indole-2-piperidinoethyl carboxylate is a potent inhibitor of Src tyrosine kinase with IC50 of 1.34 µM (11) (Figure 1).
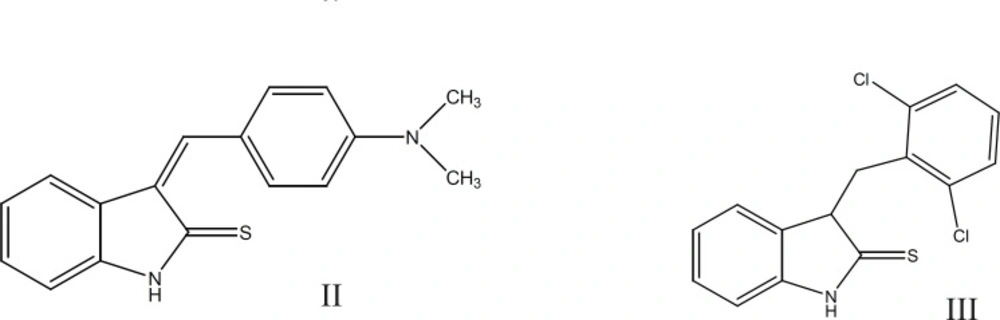
They also introduced a series of 3-(substituted-benzylidene)-1,3-dihydro-indolin-2-thione derivatives and their corresponding indolin-2-one congeners and evaluated their ability to inhibit Src PTK. In this study, (Z)-3-(4`-dimethylaminobenzylidene)-1, 3-dihydro-indolin-2-thion (II) and (Z)-3-(2`, 6`-dichlorobenzylidene)-1,3dihydro-indolin-2-thion (III), were identified as moderately active Src PTK inhibitors with IC50 of 21.91 and 21.20µM respectively (12) (Figure 2).
In an effort to find novel indole-based compounds with potential anticancer activity, a few 3-benzylidene indole-2-one and 3-phenylimino indole-2-one derivatives were synthesized and evaluated for their cytotoxic activity against HT-29 (human colon adenocarcinoma cell line) and MCF7 (human breast adenocarcinoma cell line) using short term cytotoxicity MTT assay protocol. It is proven that thyrosine kinases of the Src family (SFK) are frequently deregulated in human colorectal cancer (CRC) (13).The overexpression of thyrosine kinases in high percentages in human breast cancers is also well documented (14).
A series of thirteen 3-benzylidene indole-2-ones and 3-phenyliminoindole-2-ones (IVa-e and Va-h) were prepared in our lab as shown in (Table 1). These compounds were screened for their cytotoxic activities against colon (HT29) and breast (MCF7) cancer cell lines.
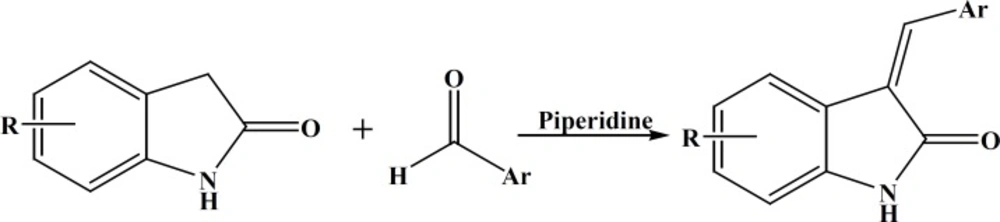
Compounds IVa-e were synthesized by condensation of appropriate indole-2-one with different aromatic aldehydes in the presence of piperidine as base (Figure 3).
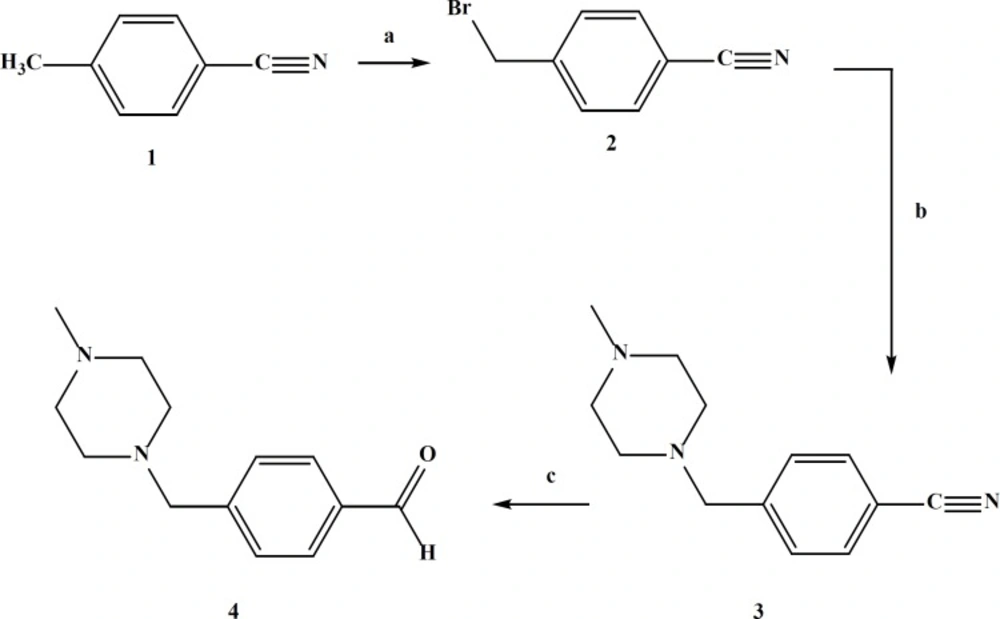
In the case of compound IVe the aldehyde was synthesized in three steps starting from 4-(bromomethyl)benzonitrile (Figure 4).
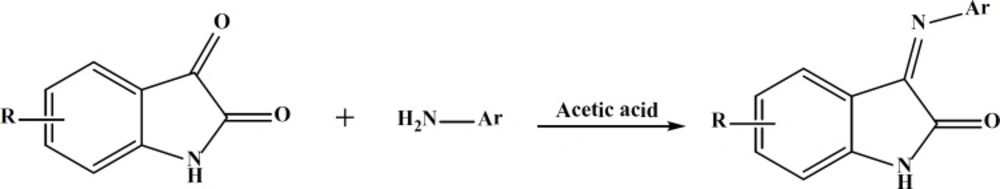
Compounds Va-h were synthesized by condensation of the appropriate isatin derivatives with the proper aromatic amines in the presence of acetic acid (Figure 5).
Experimental
Chemistry
All solvents, reagents and catalysts were of analytical grade and used without further purification. The melting points (°C) were determined by open capillary method on an Electrothermal melting point apparatus and were uncorrected. The purity of compounds was confirmed by thin layer chromatography using Whatman Sil G/UV254 silica gel plates as the stationary phase and with suitable mobile phase with fluorescent indicator, and the spots were visualized under 254 and 366 nm illumination. Infrared spectra were recorded as thin films on KBr plates with υmax in inverse centimeters. 1H NMR spectra were recorded on a Bruker DRX-Avance (500 MHz) and or (250 MHz) spectrometer using DMSO-d6 and CDCl3 as solvents and Chemical shift values are expressed in ppm (parts per million) relative to tetramethylsilane (TMS) as internal standard; s = singlet, d = doublet, dd = double doublet, t = triplet, q = quartet, m = multiplet, brs = broad singlet. Mass analyses were performed with an Agilent 6400 Series equipped with an electrospray ionization source (capillary voltage at 4000V, nebulizing gas temperature at 300 °C, nebulizing gas flow at 12 L/ min ) . All the compounds were analyzed for C, H, N, and S on a Costech model 4010 and agreed with the proposed structures within ± 0.4% of the theoretical values.
General procedure for synthesis of 3-(Substituted benzylidenyl)-indolin-2-one analogues (Compounds IVa-e)
A reaction mixture of the proper oxindole (1 equiv), aldehyde (1.2 equiv), and piperidine (0.1 equiv) in ethanol (1-2 mL/1 μmol oxindole) was stirred at 90 °C for 3-5 h (15). After the mixture cooled, the precipitate was filtered, washed with cold ethanol and hexane and recrystallized from suitable solvent to give the target compound.
4-((5-bromo-2-oxoindolin-3-ylidene)methyl)benzonitrile(IVa)
Yield 58%, mp 267-272.5 °C (dec.); ethanol; IR (KBr) υmax 3195 (NH), 2246(nitrile), 1712 (C=O), 1613 cm-1; 1H NMR (250 MHz, DMSO-d6) δ 10.86 (s,1H, NH),8.4 (d, 2H , J= 8.25 ; H-3`,5`),7.9 (m), 7.71 (s,1H, H-vinyl), 7.4 (m), 6.83 (dd, 2H, J=15, 8.25; H-2`,6`), mixture of Z and E isomers ; 13C-NMR (62.9 MHz, DMSO-d6) δ 116.2, 116.7, 116.9, 117.5, 117.9, 123.2, 123.4, 127.1, 127.9, 129.5, 131.2, 133.1, 133.3, 134.6, 136.6, 136.8, 137.3,137.8, 140, 140.7, 142.6, 143.6, 145, 147.1, 171.1, 172.4 ; ESI-MS: Observed ( M+H+ )= 325, 327 (M+Na+) = 347, 349. Calcd for C16H9BrN2O = 325.16; Anal. Found: C, 59.21; H, 2.78; Br, 24.59; N, 8.60; O, 4.91. Calcd for C16H9BrN2O: C, 59.10; H, 2.79; Br, 24.57; N, 8.62; O, 4.92%.
N-(2-fluoro-4-((2-oxoin dolin-3-ylidene)methyl)phenyl)acetamide (IVb)
Yield 15%, mp 249-252 ºC (dec.); ethanol; IR (KBr) υmax 3185 (NH), 3175(NH of acetamide), 1710 (C=O), 1660(C=O of acetamide), 1613 cm-1; 1H NMR (250 MHz, DMSO-d6) δ 10.69 (s, 1H, NH-1), 10 (s, 1H, NH of acetamide), 8.14 (dt, 1H, J= 8.5, 1.75; H-6), 7.72(m), 7.23(dd, 2H, J=15,7.5; H-5`, 6`), 6.9 (m), 2.14 (s, 3H, NHCOCH3-4`), mixture of Z and E isomers; 13C-NMR (62.9 MHz, DMSO-d6) δ 109.3, 110.1, 116.2, 116.5, 117.5, 117.8, 118.6, 119.6, 120.6, 121.1, 121.2, 122.3, 123, 124, 125.6, 126.3, 127.4, 127.5, 127.7, 128.9, 129.5, 129.7, 130.2, 130.4, 130.5, 130.7, 134.2, 140.6, 142.9, 150.5, 154.4, 167.2, 168.5, 169, 169.1; Anal. Found: C, 68.93; H, 4.41; F, 6.43; N, 9.47; O, 10.82. Calcd for C17H13FN2O2 : C, 68.91; H, 4.42; F, 6.41; N, 9.45; O, 10.80%.
N-(2-chloro-4-((2-oxoindolin-3-ylidene)methyl)phenyl) acetamide(IVc)
Yield 56%, mp 220-228 °C (dec.); ethanol; IR (KBr) υmax 3184 (N-H), 3082 (NH of acetamide), 1702 (C=O), 1662(C=O of acetamide), 1611 cm-1; 1H NMR (250 MHz, DMSO-d6) δ 10.65 (s, 1H, NH-1), 9.66 (s, 1H, NH of acetamide), 7 (m), 2.15 (s, 3H, NHCOCH3 -4`), mixture of Z and E isomers; 13C-NMR (62.9 MHz, DMSO-d6) δ 23.5, 23.6, 109.4, 110.2, 119.8, 120.6, 121.1, 121.2, 122.2, 124, 124.4, 124.6, 125, 125.4, 126.8, 127.8, 128.2, 129.1, 130.2, 130.3, 131.2, 131.5, 131.7, 132.1, 133.8, 134.6, 136, 136.5, 140.7, 142.9, 167.1, 168.5, 169; Anal. Found: C, 65.31; H, 4.2; Cl, 11.36; N, 8.95; O, 10.21. Calcd for C17H13ClN2O2: C, 65.29; H, 4.19; Cl, 11.34; N, 8.96; O, 10.23%
Preparation of 4-(bromomethyl)benzonitrile(2)
4-toluenitrile (1) (0.1 mol) was added to a flask containing N-bromosuccinimide (0.11 mol) and dibenzoyl peroxide (500 mg) in dried carbon tetrachloride (200 mL).The reaction mixture was refluxed under nitrogen atmosphere overnight. Then the mixture cooled and filtered and the filtrate was concentrated and 300 mL hexane was added to this solution to form the white crystals of 4-(bromomethyl) benzonitrile (16). The product was purified by recrystallization from chloroform. The yield was (50%), mp = 115-117 °C.
Preparation of 4-((4-methylpiperazin-1-yl)methyl)benzonitrile(3)
1-(Bromo) toluenitrile (10.2mmol) in 20 mL of chloroform was stirred at room temperature before dropwise addition of a solution of 1-methyl piperazine (28 mmol) in 5 mL chloroform. The reaction mixture was stirred at room temperature for 24 h then the reaction was quenched with water and further stirred for 30 min before extracting with chloroform. The organic layer was dried and concentrated (17). In the residue were formed crystals which were washed with hexane. It was pure 4-((4-methylpiperazin-1-yl) methyl) benzonitrile; yield (35%), mp = 65-67°C (Ref: 62-64°C); ESI-MS: Observed (M+H+) = 216. Calcd for C13H17N3 = 215.2.
Preparation of 4-((4-methylpiperazin-1-yl) methyl)benzaldehyde (4) (18)
4-((4-methylpiperazin-1-yl)methyl)benzonitrile (9 mmol)was dissolved in formic acid 75% (37 mL) and raney nickel alloy (2 g) was added to this solution. The mixture was refluxed for 2 h then filtered with celite and washed with 20 mL of cold ethanol 96°.The filtrate was concentrated and again filtered to remove the green colloidal impurities to give (1.8 g) crude product, ESI-MS: Observed (M+H+) = 219. Calcd for C13H18N2O = 218.29
Synthesis of 3-(4-((4-methylpiperazin-1-yl)methyl)benzylidene)indolin-2-one (IVd)
A reaction mixture of oxindole (1 equiv), 4-((4-methylpiperazin-1-yl) methyl)benzaldehyde (1.2 equiv), and piperidine (0.1 equiv) in ethanol (1-2 mL /1 μmol oxindole) was stirred at 90 °C overnight. The solvent of the reaction mixture was evaporated and the residue was dissolved in warm ethyl acetate and passed through a column of silica gel. The polarity of eluting solvent was increased with the addition of methanol to the ethyl acetate. The yellow liquid phase was collected and the solvent was evaporated to achieve 3-(4-((4-methylpiperazin-1-yl)methyl)benzylidene) indolin-2-one: Yield 38%, mp 264-269 °C (dec.) ; ethanol; 1H NMR (500 MHz, DMSO-d6) δ 10.58 (s, 1H, NH-1), 7.66 (d, 2H, J= 8, H-2′,6′), 7.59 (s , 1H, H-vinyl),7.56(d, J= 8, 1H, H-4), 7.43(d, 2H, J= 8, H-3‹, 5`), 7.21(t, 1H, J= 7.5, H-6), 6.85 (m, 2H, H-5,7),3.52 (s, 2H, CH2), 2.37(m, 8H, CH2 piperazine), 2.16(s, 3H, CH3) ; 13C-NMR (62.9 MHz, DMSO-d6) δ 45.2, 52.1, 54.3, 61.5, 110.1, 120.8, 121.1, 122.3, 127.1, 128.5, 129, 129.2, 130, 131.8, 132.9, 135.7, 140.2, 142.8, 164.6, 168.6; ESI-MS: Observed ( M+H+ ) = 334. Calcd for C21H23N3O = 333.43; Anal. Found: C, 75.70; H, 6.93; N, 12.58; O, 4.78.Calcd for C21H23N3O: C, 75.65; H, 6.95; N, 12.60; O, 4.80%.
3-((5-(4-fluorophenyl)pyridin-3-yl)methylene)indolin-2-one(IVe)
Yield 85%, mp 204-206.9 ºC (dec.); ethanol; IR (KBr) υmax 3160 (N-H), 1720 (C=O), 1689, 1607 cm-1; 1H NMR (250 MHz, DMSO-d6) δ 10.70 (s, 1H, NH), 9.23 (t,1H, J= 2; H-2′), 9.15 (d,1H, J=1.75; H-6′),8.9 (m,1H, H-4′), 7.91 (s, 1H, H-vinyl), 7.84 (m), 7.72 (t, 1H, J = 7.5 Hz; H-4), 7.39 (m), 7.26 (t, 1H, J =7.5; H-5), 7.05 (t,1H, J =7.5; H-6), 6.87 (m,1H; H-7) , mixture of Z and E isomers ; 13C-NMR (62.9 MHz, DMSO-d6) δ 115.9, 116.2, 120.2, 120.7, 121.3, 122.1, 124.3, 128.9, 129, 129.1, 129.6, 129.8, 130.6, 130.7, 131.8, 132.4, 132.7, 132.8, 133.3, 133.7, 134.3, 135.6, 141.1, 143.2, 147.9, 148, 148.1, 150.9, 160.5, 164.4, 167, 168.1; ESI-MS: Observed ( M+H+ ) = 317. Calcd for C20H13FN2O = 316.3. Anal. Found: C, 75.96; H, 4.15; F, 6.03; N, 8.84; O, 5.05. Calcd for C20H13FN2O: C, 75.94; H, 4.14; F, 6.01; N, 8.86; O, 5.06%.
General Procedure for Compounds (Va-h)
A mixture of indole-2, 3-dione (0.01M) and Amine (0.01M) in absolute ethanol(20 mL) was refluxed for 20 h in the presence of 2-3 drops of glacial acetic acid (19). After cooling, the resultant was filtered and washed with hexane and recrystallised from appropriate solvent to give compounds Va-h.
5-fluoro-3-(p-tolylimino)indolin-2-one (Va)
Yield 38%, mp 264-269 ºC (dec.) ; ethanol; IR (KBr) υmax 3256 (N-H), 1741 (C=O),1652 (C=N) ,1613 cm-1; 1H NMR (250 MHz, DMSO-d6) δ 11.03 (s,1H, NH-1), 7.15 (m, 6H, H-6,7,2′,3′,5′,6′), 6.14 (dd, 1H, J = 8.5, 2.5 Hz; H-4), 2.37(s, 3H, CH3-4′), mixture of Z and E isomers; 13C-NMR (62.9 MHz, DMSO-d6) δ 20.5, 111.5, 111.9, 112.5, 112.6, 115.9, 116, 117.4, 119.8, 120.5, 120.9, 128.7, 130, 134.6, 143.2, 147.2, 154.3, 154.8, 158.5, 163.5; ESI-MS: Observed (M+H+) = 255 Calcd for C15H11FN2O = 254.2;Anal. Found: C, 70.89; H, 4.31; F, 7.45; N, 11.03; O, 6.27. Calcd for C15H11FN2O: C, 70.86; H, 4.36; F, 7.47; N, 11.02; O, 6.29%.
5-fluoro-3-(4-hydroxyphenylimino)indolin-2-one (Vb)
Yield 74%, mp 307-320 ºC (dec.) ; ethanol; IR (KBr) υmax 3298, 1711 (C=O), 1619, 1599 (C=N) cm-1; 1H NMR (250 MHz, DMSO-d6) δ 10.97 (s,1H, NH-1), 9.66 (s, 1H, OH-4′), 7.26 (m),6.84 (m), 6.44 (dd,1H, J =8.5, 2.5 Hz; H-4), mixture of Z and E isomers ; 13C-NMR (62.9 MHz, DMSO-d6) δ 108.6, 109, 111.1, 111.3, 111.5, 112.3, 112.4, 114.7, 115.9, 116, 116.2, 119, 119.4, 119.9, 120.1, 120.5,124.6, 138.8, 140.7, 141, 142.9, 143, 153.4, 154.8, 155.8, 156.9, 158.6, 158.9, 163.7; ESI-MS: Observed ( M+H+ ) = 257. Calcd for C14H9FN2O2 = 256.23; Anal. Found: C, 65.68; H, 3.53; F, 7.42; N, 10.91; O, 12.47.Calcd for C14H9FN2O2: C, 65.62; H, 3.54; F, 7.41; N, 10.93; O, 12.49%.
4-(5-fluoro-2-oxoindolin-3-ylideneamino)benzamide(Vc)
Yield 19%, mp 277-310 °C (dec.) ; ethanol; IR (KBr) υmax 3394 (NH2), 3232 (N-H), 1741 (C=O Oxindole),1673 (C=O benzamide), 1630 (C=N) cm-1; 1H NMR (250 MHz, DMSO-d6) δ 11.09 (s,1H, NH-1), 7.94(m), 7.33 (m),7.06 (m), 6.92 (m), 5.97(dd, 1H, J= 8.25, 2.5; H-4), mixture of Z and E isomers; 13C-NMR (62.9 MHz, DMSO-d6) δ 109.7, 110.1, 111.7, 112.1, 112.7, 112.8, 115.8, 115.9, 116.9, 118.2, 120.9, 121.3, 127.9, 129.2, 130.8, 143.4, 152.4, 163.3, 167.3, 167.6 ; ESI-MS: Observed ( M+H+ ) = 284. Calcd for C15H10FN3O2 = 283.2; Anal. Found: C, 63.70; H, 3.55; F, 6.72; N, 14.85; O, 11.33. Calcd for C15H10FN3O2 : C, 63.60; H, 3.56; F, 6.71; N, 14.83; O, 11.30%.
3-(4-chlorophenylimino)-5-(trifluoromethoxy)indolin-2-one (Vd)
Yield 43%, mp 230-240 ºC (dec.); ethanol; IR (KBr) υmax 3282 (N-H), 1746 (C=O),1628 (C=N) cm-1; 1H NMR (250 MHz, DMSO-d6) δ 11.21 (s,1H, NH-1), 7.46 (m), 7.06 (m), 6.20 (d,1H, J= 1.25 Hz; H-4), mixture of Z and E isomers ; 13C-NMR (62.9 MHz, DMSO-d6) δ 112, 112.7, 116, 117.8, 119.3, 121.1, 127.3, 127.4, 128.2, 129.4, 129.5, 142.1, 143.3, 144.7, 146, 147.3, 148.7, 152.9, 154.8, 158.4, 163.3. 6 ; ESI-MS: Observed ( M+H+ ) = 342. Calcd for C15H8ClF3N2O2 = 340.7; Anal. Found C, 52.92; H, 2.36; Cl, 10.43; F, 16.75; N, 8.21; O, 9.37. Calcd for C15H8ClF3N2O2 : C, 52.88; H, 2.37; Cl, 10.41; F, 16.73; N, 8.22; O, 9.39%.
3-(p-tolylimino)-5-(trifluoromethoxy)indolin-2-one (Ve)
Yield 59%, mp 266.5-268.5 ºC (with dec.); ethanol; IR (KBr) υmax 3254 (N-H), 1741 (C=O),1619(C=N) cm-1; 1H NMR (250 MHz, DMSO-d6) δ 11.3 (s,1H, NH-1), 7.15(m, 6H, H- 6, 7, 2′, 3′, 5′, 6′), 6.25 (d, 1H, J = 1.25 Hz, H-4), 2.36 (s, 3H, CH3-4′), mixture of Z and E isomers; 13C-NMR (62.9 MHz, DMSO-d6) δ 20.41, 20.55, 111.8, 112.6, 116.1, 117.3, 117.8, 119.9, 127.1, 128.7, 129.9, 134.7, 142.1, 145.7, 147.4, 154.1, 163.4 ; ESI-MS: Observed ( M+H+ ) = 321.Calcd for C16H11F3N2O2 = 320.2; Anal. Found: C, 60.2; H, 3.45; F, 17.81; N, 8.74; O, 9.97. Calcd for C16H11F3N2O2 : C, 60.00; H, 3.46; F, 17.80; N, 8.75; O, 9.99%.
3-(4-hydroxyphenylimino)-5-(trifluoromethoxy)indolin-2-one (Vf)
Yield 80%, mp 230-238 °C (dec.); ethanol; IR (KBr) υmax 3314 (O-H), 3209 (N-H), 1732 (C=O),1627 (C=N) cm-1;1H NMR (250 MHz, DMSO-d6) δ 11.13 (1H,s, NH-1), 9.66 (s,1H, OH-4′), 7.37 (m), 6.89(m), 6.60 (d, 1H , J = 1.25 Hz; H-4), mixture of Z and E isomers; 13C-NMR (62.9 MHz, DMSO-d6) δ 111.5, 112.4, 114.7, 115.9, 116.3, 117.5, 119.9, 123.8, 124.8, 125.8, 126.8, 138.7, 141, 142.1, 143.4, 145.6, 149.1, 153.2, 155.9, 157.1, 158.9, 163.7; ESI-MS: Observed ( M+H+ ) = 323.Calcd for C15H9F3N2O3 = 322.2; Anal. Found: C, 55.93; H, 2.84; F, 17.67; N, 8.69; O, 14.92. Calcd for C15H9F3N2O3: C, 55.91; H, 2.82; F, 17.69; N, 8.69; O, 14.90%.
4-(2-oxo-5-(trifluoromethoxy)indolin-3-ylideneamino)benzamide (Vg)
Yield 49%, mp 296-304 °C (dec.); ethanol; IR (KBr) υmax 3429 (NH2), 3121 (N-H), 1727 (C=O Oxindole),1697 (C=O benzamide), 1608 (C=N) cm-1 ;1H NMR (250 MHz, DMSO-d6) δ 11.22 (brs,3H, NH-1, CONH2-4′), 7.48 (m,6H, H-6,7,2′, 3′, 5′, 6′), 6.10 (s,1H, H-4), mixture of Z and E isomers ; 13C-NMR (62.9 MHz, DMSO-d6) δ 112, 112.7, 116, 116.8, 118, 118.3, 121.8, 122.1, 127.4, 127.9, 129.1, 130.1, 130.8, 142.1, 143.3, 144.8, 145.9, 151.5, 152.5, 154.4, 158.4, 163.2, 167.1, 167.6; ESI-MS: Observed ( M+H+ ) = 350. Calcd for C16H10F3N3O3= 349.26; Anal. Found: C, 55.05; H, 2.88; F, 16.30; N, 12.05; O, 13.72. Calcd for C16H10F3N3O3: C, 55.02; H, 2.89; F, 16.32; N, 12.03; O, 13.74%.
4-(5, 7-dichloro-2-oxoindolin-3-ylideneamino)benzamide (Vh)
Yield 58%, mp 322-325 °C (dec.); ethanol; IR (KBr) υmax 3460 (NH2), 3339 (N-H), 1733(C=O Oxindole), 1662(C=O benzamide),1610(C=N) cm-1; 1H NMR (250 MHz, DMSO-d6) δ 11.65 (s,1H, NH-1), 11.51 (s,2H, CONH2-4′), 7.3 (m), 6.18 (d, 1H, J =1.75 Hz; H-4), mixture of Z and E isomers ; 13C-NMR (62.9 MHz, DMSO-d6) δ 112.4, 115.8, 116.6, 116.9, 117.9, 118.4, 120.6, 120.8, 121.3, 122.8, 123.3, 123.9, 125.6, 126.8, 127, 127.9, 129, 129.2, 130.3, 131, 133, 135, 142.2, 143.6, 146.5, 151.2, 151.6, 152, 153.4, 158.1,159.4, 163, 167.2, 167.5, 168; ESI-MS: Observed ( M+H+ ) = 335.Calcd for C15H9Cl2N3O2 = 334; Anal. Found: C, 53.91; H, 2.70; Cl, 21.24; N, 12.58; O, 9.59. Calcd for C15H9Cl2N3O2 : C, 53.91; H, 2.71; Cl, 21.22; N, 12.57; O, 9.58%.
| Comp. ID. | MCF-7a | HT29b | ||
|---|---|---|---|---|
| IC50(µM) | RSD% | IC50(µM) | RSD% | |
| IVa | 6 | 7.9 | 13.13 | 4.3 |
| IVb | 9.3 | 5.0 | 10.75 | 6.8 |
| IVc | >100 | - | 38.27 | 4.3 |
| IVd | 42.07 | 3.8 | 7.51 | 6.9 |
| IVe | 8.54 | 6.7 | >100 | - |
| Va | >100 | - | >100 | - |
| Vb | >100 | - | >100 | - |
| Vc | >100 | - | >100 | - |
| Vd | >100 | - | 90.31 | 2.1 |
| Ve | >100 | - | >100 | - |
| Vf | >100 | - | >100 | - |
| Vg | 27.2 | 7.1 | 61.9 | 3.4 |
| Vh | 65.86 | 8.1 | >100 | - |
| TAMOXIFEN | 6.05 | 4.9 | 10.36 | 3.7 |
Cytotoxic activity
Cell lines
HT-29(human colon adenocarcinoma cell line) and MCF-7 cells (human breast adenocarcinoma cell line) were used for the in-vitro screening of newly synthesized compounds. Cell lines were obtained from Pasture Institute of Iran, Tehran, Iran. Each cell line was cultured in suitable medium for desired growth, plus 10% FBS and 1% penicillin-streptomycine in a humidified incubator at 37°C in an atmosphere of 95% O2 and 5% CO2. Then the growth curve of each cell line was plotted.
Subculture of adherent cell lines
Cultures were observed using an inverted microscope to assess the degree of confluency and the absence of bacterial and fungal contaminants was confirmed. Cell monolayer was washed with PBS (Phosphate buffer solution) using a volume equivalent to half the volume of culture medium. Trypsin was added on to the washed cell monolayer, flask was rotated to cover monolayer with trypsin. Flask was returned to the incubator and left for 2-10 min. The cells were examined using an inverted microscope to ensure that all the cells were detached and floated. The cells were resuspended in a small volume of fresh serum containing appropriate medium.A 100 μL aliquot was removed to perform a cell count. The required number of cells were transferred to plates (tissue culture grade, 96 wells, round bottom) and incubated for 24 h at 37 °C and 5 % CO2.
| Compound | CLogP a | P b | V c | SAd | DMe |
|---|---|---|---|---|---|
| IVa | 3.7836 | 1057.02 | 81.58 | 330.762 | 4.144 |
| IVb | 3.7808 | 5454.61 | 92.26 | 123.949 | 2.146 |
| IVc | 2.0496 | 2139.00 | 81.36 | 300.439 | 4.293 |
| IVd | 2.3696 | 3488.20 | 85.56 | 118.548 | 3.918 |
| IVe | 3.538 | 5205.99 | 103.14 | 192.032 | 3.159 |
| Va | 3.292 | 926.57 | 71.75 | 571.2 | 1.158 |
| Vb | 2.126 | 1049.3 | 67.67 | 133.9 | 2.338 |
| Vc | 1.306 | 1166.7 | 75.06 | 249.2 | 5.4 |
| Vd | 4.391 | 1648.15 | 78.9 | 235.2 | 2.44 |
| Ve | 4.177 | 1725.83 | 80.2 | 203.6 | 2.044 |
| Vf | 3.011 | 1747.35 | 76.11 | 216.02 | 2.957 |
| Vg | 2.191 | 1456.59 | 83.51 | 128.59 | 5.53 |
| Vh | 2.589 | 1233.72 | 83.87 | 451.84 | 4.027 |
MTT assay
Cell viability was assessed by MTT assay (microculture tetrazolium/formazan assay). After 24 h the medium of the cells which were seeded in 96 well plates was replaced by fresh medium containing compounds, each experiment was done in triplicate and six concentrations (20, 21). Tamoxifen was used as positive control. Then, the cells were incubated at 37°C for 72 h, the medium was changed by fresh medium containing MTT ([3-(4, 5- dimethylthiazol-2-yl)-2,4-diphenyltetrazolium bromide]) with a final concentration of 0.5 mg/mL. The cells were incubated for another 4 h in a humidified atmosphere at 37°C and after that the medium containing MTT was removed and remaining MTT formazan crystals were dissolved in 200 μL DMSO. The absorbance was measured at 570 nm after 20 min shaking using an ELISA reader. IC50 was defined as the concentration of the compounds that produced a 50% decrease in cell viability relative to the negative control which was wells exposed to the solvent without any compound. IC50 was determined by plotting a graph of Log (concentration of compound) vs % effect (cell viability). A line drawn from 50 % value on the Y axis meets the curve and interpolate to the X axis. The X axis value gives the Log (concentration of compound). The antilog of that value gives the IC50 value.
Results and Discussion
All the synthesized compounds were assayed for their cytotoxic activities against colon cancer cell lines (HT29) and breast cancer cell lines (MCF7). The results are presented in Table 2.
As it appears from Table 2, the most active compounds are among the 3-benzylidene indole-2-one derivatives (IVseries). In 3-phenyliminoindole-2-ones (V series) only compound Vg showed moderate cytotoxic activity (27.2 µM against MCF7 and 61.9 µM against HT29). Compounds IVa and IVb in 3-benzylidene indole-2-one series showed the best activities against both MCF7and HT29 cell lines. The most potent compound against MCF7 breast cancer cell is compound IVa which bears 5-bromo substitution. This is in complete agreement with the report by Olgen et al for a series of N-benzyl-indole derivatives. They have found the 5-bromo substituted derivatives as the most potent inhibitors of c-Src tyrosine kinase.
Compound IVd which shows highest activity against HT29 (7.51 µM) has a moderate activity (42.07 µM) against MCF7.
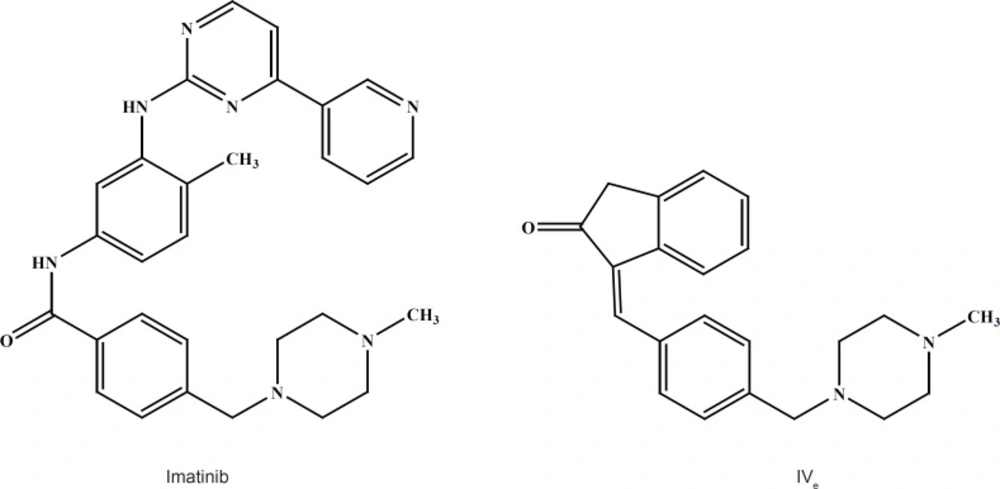
Compound IVd is a 3-benzylidene indole-2-one derivative with a methyl piperazine moiety similar to the structure of Imatinib which is a 2-phenylaminopyrimidine derivative that functions as a specific inhibitor of a number of tyrosine kinase enzymes (Figure 6).
Global physicochemical properties for compounds IVa-e and Va-h were calculated using Chemdraw Ultra version 8.0 and the results are presented in Table 3.
Efforts to find a relationship between these physicochemical parameters and cytotoxic activities of the compounds did not result in a clear correlation. The only interesting point in this regard is the closeness of CLogP values for the two most active compounds IVa and IVb which are 3.7836 and 3.7808 respectively.
Studies are in progress to examine whether further structural modifications, specialy with regard to substituents at the 5 position, can result in enhancement of the cytotoxic activity of 3-benzylidene indole-2-one derivatives. Since MTT assay is a general screening method to evaluate the cytotoxicity of compounds and lacks specificity for anti-cancer evaluation, further mechanistic studies are needed to demonstrate the anti cancer and anti apaptosis activity of these compounds.
Src family of thyrosine kinases and other members of kinase enzymes which are proven to be involved in human colon and/or breast cancer are among the best candidates for the future enzyme inhibition studies.