Introduction
Liver, which is involved in almost all of the biochemical pathways in the body, plays a vital role in maintaining, performing and regulating its homeostasis. Therefore, a healthy liver is necessary for the health and well-being. Unfortunately, liver is often abused by environmental toxins, poor eating habits and alcohol and medications and over the counter drug use, which can damage and weaken the liver and eventually lead to hepatitis, cirrhosis and other liver diseases (1). Modern medicine has little to offer to alleviate hepatic diseases and there are not many drugs available to treat liver disorders. Hence, many folk remedies of plant origin have been evaluated according to their possible hepatoprotective effects against the liver damage in experimental animals. Silymarin is a polyphenolic component isolated from the fruits and seeds of Silybum marianum (2, 3). It restores the GSH content and facilitates the ATPase activity and promotes RNA polymerase I in hepatocytes (4). Flavonolignans isolated from silymarin are known to lead to regeneration of liver tissue (5) and hepatic membrane stabilization response (6). Silymarin has a great potency for hepatoprotection against toxic agents like CCl4 and were used here as a reference drug.
CCl4-induced hepatotoxicity model is frequently used to investigate the hepatoprotective effects of drugs and plant extracts. The changes associated with CCl4-induced liver damage are similar to that of acute viral hepatitis (7).
The family Lamiaceae is one of the largest and most distinctive families of flowering plants with about 220 genera and almost 4000 species worldwide (8). Many biologically active essential oils have been isolated from various members of this family so far. The genus of Otostegia is a member of this family which is comprised of 20 species that are distributed over the east of Asia, from them Otostegia persica (Burm.) Boiss (O. persica ) locally called «Golder» is endemic to south of Iran. This is a spiny shrub plant, with about 1.5 m height and with rectangular woody stems. Its leaves are opposite on stems with short petiole and obovate blade and covered with dense white hairs. Flowers have funnel-shaped calyx with longitudinal ridges and bilabiate white corolla with hairy upper lip (9).
The flowers of the plant are widely used as an additive to yoghurt, butter, milk and meat. It has also been used in Iranian traditional medicine as analgesic in toothache and arthritis. Hydroalcoholic extract of O. persica alleviates the morphine withdrawal syndrome (10). O. persica extracts (methanolic, chloroform and hexane) showed antimicrobial activities against the Gram-positive strains (11). The aqueous extract of the aerial parts of the plant has been used as antispasmodic, antihistaminic and antiarthritic (12). Phytochemical studies on this plant resulted in the isolation and characterization of geraniol, eugenol, ceryl alcohol, hentriacontane, caffeic acid, p- hydroxybenzoic acid, β-sitosterol, β-sitostery acetate, β-amyrin, campesterol and stigmasterol (13). Oral administration of ethanol extract of O. persica for 21 days showed antidiabetic effect in rats (14). It has been reported that its ethanolic extract has anti-glycation property which belongs to the known compound 3, 7-dihydroxy-4’, 6, 8-trimethoxy-flavone (15). It has strong antioxidant property and our recent studies indicated that the methanolic extract of its aerial parts has anti-diabetic effect through the stimulation of insulin release and pancreas tissue improvement (16, 17). In addition, it can decrease the hepatic dysfunction originated from diabetes mellitus (18). In this study, we aimed to evaluate the hepatoprotective effect of the methanol extract of O. persica shoot on liver injury model in rats.
Experimental
Plant material extraction procedure
The aerial parts of the O. persica were collected from Jiroft, Kerman, southeastern of Iran, taxonomically identified and approved by Dr. SM. Mirtadzaddini, Biology Department of Shahid Bahonar University of Kerman (voucher number : 40642, deposited in : Herbarium of Tehran University, director: Dr. F. Attar). The O. persica was powdered in an electrical grinder. The extraction was carried out through the maceration of dry plant powder in methanol 80% for 48 h at room temperature. Then, it was submitted to the extraction with methanol by soxhlation. After the extraction, methanol was evaporated by rotary evaporator at 40-50ºC and was dried using a freeze-dryer at - 50ºC. The yield of extraction was 10%. The extract was prepared in distilled water before use.
Laboratory animals
Adult male Wistar rats with an average weight of 200-220 g were used in this assay. They were purchased from the animal breeding laboratories of Pasteur Institute (Tehran, Iran) and had free access to food and water, and were maintained in a controlled temperature (24 ± 2ºC) and light cycle (12 h light and 12 h dark).
Experimental design
The animals were divided into 6 groups, each consisting of 6 rats orally treated with 50% CCl4 in liquid paraffin (2.5 mL/Kg bw, per os) 60 min after the administration of O. persica methanol extract (in 200, 300, 400 mg/Kg bw doses), Legalon which contained 70% silymarin (420 mg/Kg bw) as a reference drug and 0.5 mL distilled water. One group of normal (untreated) rats was also used in our study. Throughout the experiments, local ethical guidelines were considered for taking care of laboratory animals. Twenty-four hours after the CCl4 administration, rats were sacrificed by overdose of diethyl ether and blood samples were withdrawn, collected in heparinized tubes and were centrifuged at 3000 × g for 10 min to obtain plasma. Plasma samples were used to determine the lipid peroxidation level as well as to test the aspartate aminotransferase (AST) and alanine transaminase (ALT) activities. On the other hand, the liver of each rat was promptly removed and used to determine the tissue levels of malondialdehyde (MDA) and glutathione (GSH).
Biochemical assays
Pars Azmoon standard kits and RA-1000 Autoanalyzer were used to measure the AST and ALT activities in plasma. The methodology described by Kurtel et al. (19) was used to determine the plasma lipid peroxidation level. Besides, rats were sacrificed using diethyl ether to determine the lipid peroxidation in liver tissue. The liver of each rat was immediately excised and chilled in ice-cold 0.9% NaCl and then perfused via the portal vein with ice-cold 0.9% NaCl. After washing with 0.9% NaCl, the method of Ohkawa et al. (20) modified by Jamall and Smith (21) was used to determine the lipid peroxidation in tissue samples. The evaluation of cellular GSH in liver tissue was determined by Sedlak and Lindsay method (22).
Histopathological studies
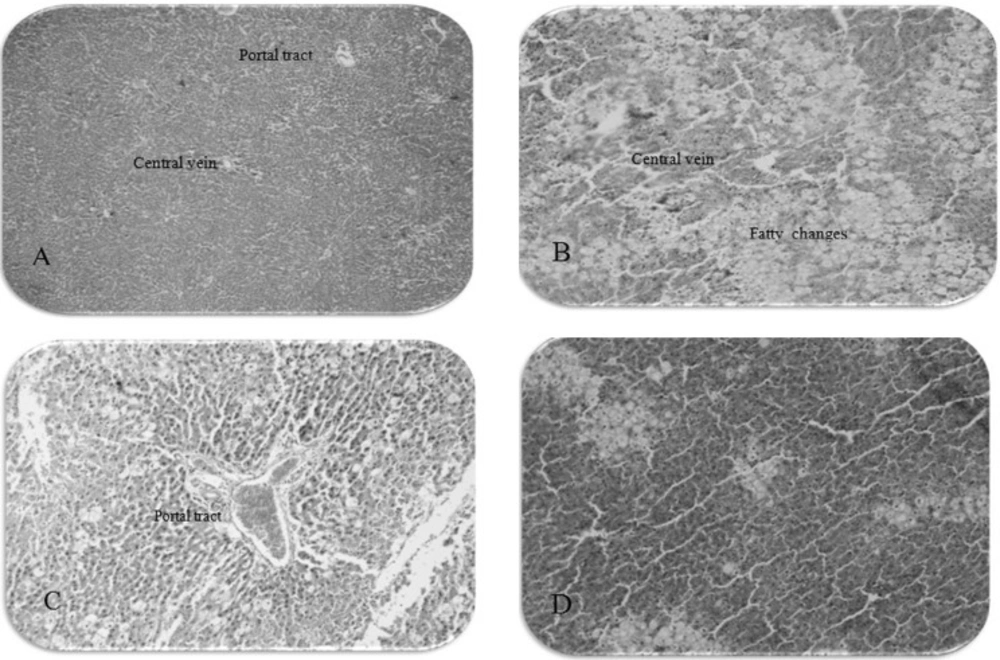
For the histopathological study, the livers of six animals in each group were immediately removed and the tissues were fixed in 10% formalin for a period of at least 24 h. The paraffin sections were then prepared (Automatic Tissue Processor, Lietz, 1512) and cut into 5 μm thick sections in a rotary microtome. Thereafter, the sections were stained with haematoxylin-eosin dye and mounted in Canada balsam. The histopathological slides were examined and photographs were taken with a photomicroscope. Histological damage was expressed using the following score system: Ø: absent; ⊥: minimal; +: mild; + +: moderate; + + +: severe.
Statistical analysis
The obtained data were analyzed by one-way ANOVA followed by the Tukey’s post-hoc test and p < 0.05 was considered statistically significant.
Results and Discussion
The effects of the methanol extract of O. persica on biochemical parameters of rats intoxicated by carbon tetrachloride (CCl4) were evaluated in this study. CCl4 was found to cause several fold increases in plasma AST (2561.82%) and ALT (3206.53%) levels (Table 1).
| Materials | Dose | ALT | % changea | AST | % changea | |
|---|---|---|---|---|---|---|
| Control | 99.5 ± 3.8 | - | 165 ± 12.8 | - | ||
| CCl4b | 2.5c | 3290 ± 215.8*** | + 3206.53 | 4392 ± 129.5*** | + 2561.82 | |
| Legalond | 420 | 1434 ± 178.5*** | – 56.41 | 2533 ± 178.5*** | – 42.33 | |
| Extractd | 200 | 3035 ± 122.3 | – 7.75 | 4119 ± 191.6 | – 6.21 | |
| 300 | 2140 ± 249.2** | – 34.95 | 2996 ± 104.5*** | – 31.78 | ||
| 400 | 2857 ± 149.5 | – 13.16 | 3883 ± 49 | – 11.59 | ||
Moreover, the liver (117.21%) and plasma (345.77%) lipid peroxidation levels were increased significantly in CCl4-treated group compared with those in the normal group which has been evidenced by MDA determination. However, the content of GSH in the liver was decreased in CCl4-treated group (51.5%). The plasma (26.6%) and liver (22.96%) MDA level, as well as the plasma ALT (34.95%) and AST (31.78%) were significantly reduced in rats that received a dose of 300 mg/Kg of the methanol extract of the aerial parts of O. persica. On the other hand, the content of GSH in the liver tissue was increased significantly (52.5%) by administering 300 mg/Kg of the extract (Tables 1, 2).
| Material | Dose | Plasma MDA level | % changea | Liver MDA level | % changea | Liver GSH level | % change |
|---|---|---|---|---|---|---|---|
| Control | - | 0.673 ± 0.02 | - | 313 ± 17.4 | - | 114.32 ± 5.17 | - |
| CCl4b | 2.5c | 3 ± 0.07*** | + 345. 77 | 679.86 ± 14.9*** | + 117.21 | 55.45 ± 3.7*** | – 51.5 |
| Legalond | 420 | 1.9 ± 0.35** | – 36.67 | 445.26 ± 34.4** | – 34.5 | 91.63 ± 6.56*** | + 65.2 |
| Extractd | 200 | 2.9 ± 0.07 | – 3.3 | 627.4 ± 44.9 | – 7.72 | 62.47 ± 2.38 | + 12.7 |
| 300 | 2.2 ± 0.24* | – 26.6 | 523.78 ± 31.5* | – 22.96 | 84.55 ± 7.2** | + 52.5 | |
| 400 | 2.8 ± 0.03 | – 6.66 | 602.03 ± 52.1 | – 11.45 | 67.06 ± 3.85 | + 20.9 |
Histopathological examination of the liver sections confirmed that the normal liver architecture was damaged with CCl4 administration. However, the pretreatment of methanol extract at 300 mg/Kg significantly lessened the severity of histopathological injury compared with the CCl4 group (Table 3 and Figure 1).
| Microscopic observation | Control | CCl4 | Legalon | Extract | Extract | Extract |
|---|---|---|---|---|---|---|
| Degeneration in hepatocytes | Ø | + + + | ⊥ | + + | + | + + |
| Degeneration in hepatic cords | Ø | + + | + | + + | + | + |
| Deformation in hepatocytes | Ø | + | ⊥ | + | + | + |
| Focal necrosis | Ø | + | Ø | ⊥ | Ø | Ø |
| Congestion in central vein | ⊥ | + + | + | + + | + | + + |
| Congestion in sinusoids | Ø | + | + | + | + | + |
| Infiltration of lymphocytes | + | + + + | + | + + + | + | ++ |
| Kupffer cells proliferation | Ø | + | Ø | ⊥ | ⊥ | ⊥ |
| Bleeding area in hepatic lobes | Ø | Ø | Ø | Ø | Ø | Ø |
Our results indicate that the methanol extract of O. persica exerts hepatoprotective properties against CCl4-induced liver damage. CCl4 is a well-known hepatotoxin and the exposure to this chemical is known to induce the oxidative stress and cause the liver injury through free radicals formation (23). The changes associated with CCl4-induced liver damage are similar to those of acute viral hepatitis (7). In accordance with our findings, it has been shown that the liver of CCl4-intoxicated rats has been exerted the massive fatty change, gross necrosis, broad infiltration of lymphocytes and kupffer cells around the central vein and loss of cellular boundaries (24-28). CCl4-induced hepatotoxicity is believed to include two phases. The initial phase involves the metabolism of CCl4 by cytochrome P450, which leads to the formation of free radicals (CCl3●, CCl3OO●) and lipid peroxidation (29). The second step involves the activation of kupffer cells, probably through free radicals. The activation of kupffer cells is accompanied by the production of proinflammatory mediators (30). As a result of the hepatic injury, the altered permeability of the membrane causes the enzymes from the cells to be released into the circulation which damages the hepatic cells, as shown through the abnormally high level of serum hepatospecific enzymes. Free radicals also affect the antioxidant defense mechanisms, reduce the intracellular concentration of GSH and decrease the activity of SOD and CAT. Lipid peroxidation is a chain reaction that involves the oxidation of polyunsaturated fatty acids in membranes induced by free radicals and is an indicator of oxidative cell damage. Direct measurement of oxidative stress in humans is difficult since the active oxygen species and free radicals are extremely short-lived (31). Instead, products of the oxidative process are measured. The elevation of MDA levels, which is one of the end products of lipid peroxidation in the liver, and the reduction of hepatic GSH levels are important indicators in CCl4-intoxicated rats (32). Glutathione exists in reduced (GSH) and oxidized (GSSG) states. GSH can be regenerated from GSSG through the enzyme glutathione reductase. In healthy cells and tissues, more than 90% of the total glutathione pool is in the reduced form (GSH) and less than 10% exists in the disulfide form (GSSG). An increased GSSG-to-GSH ratio is considered as the indicative of oxidative stress (33).
Two compounds of O. persica methanol extract which were separated by column and paper chromatography showed significant antioxidant activities compared with butylated hydroxyl anisole (BHA) and alpha tocopherol. These active compounds were identified as morin and quercetin (34). It is thought that antioxidants play a significant role in protecting the living organisms from the toxic effects of chemical substances such as CCl4 and carcinogens (35). Morin has been shown to act as a potent antioxidant (36), xanthine oxidase inhibitor (37) and modulator of lipoxygenase and cyclooxygenase activities in the arachidonic acid cascade (38). Morin prevents acute liver damage via inhibiting the production of TNF-α, IL-6 and iNOS (39). Besides, Quercetin, a natural antioxidant, reveals its antioxidant properties through inhibiting the lipid peroxidation via blocking the enzyme xanthine oxidase (40), and directly scavenging hydroxyl, peroxy and superoxide radicals (41). Quercetin also potentiates an antioxidative defense mechanism through increasing the absorption of vitamin C (42) and inhibiting the structural damage to the proteins (43).
Conclusion
The methanol extract of O. persica has protective effect against the acute liver damage and hepatoprotective mechanisms of this extract on CCl4-induced acute liver damage might be due to the decreased lipid peroxidation (decreased MDA level and increased content of GSH). More studies are needed to determine further mechanisms involved in the hepatoprotective effects of this plant.