Introduction
Reactive oxygen species (ROS) in the forms of superoxide anions, hydroxyl radicals and hydrogen peroxide are generated from the auto-oxidation of lipids, as well as reactive nitrogen species (RNS) (1). Formations of these excess ROS and RNS by Ultraviolet (UV) irradiation, smoking and drug metabolisms are likely to damage several cellular components such as lipids, proteins, nucleic acids, and DNAs through the oxidation or nitration processes (2). In addition, these reactive oxygen species cause inflammation or lesions on various organs and are associated with various degenerative diseases, including cancer, ageing, arteriosclerosis, and rheumatism (3).
All aerobic organisms, including human beings, have antioxidant defences that protect against oxidative damages, numerous damage removal and repair enzymes to remove or repair damaged molecules (4). However, these natural antioxidant mechanisms can be inefficient (5-8). Although some synthetic antioxidant, such as butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) are commonly used in processed foods, they have been reported to have some side effects (9-11). Therefore, recent search to find natural originated antioxidant has been increased.
Nerium oleander L. is an evergreen shrub or small tree in the dogbane family Apocynaceae and is planted throughout the tropical region of the world as garden and roadside trees. Previously cardiotonic, antibacterial, anticancer and anti platelet aggregation activities and also its depression in the central nervous system have been reported from these species (12, 13). Flavonoids are a class of compounds isolated in large quantities from genus of Nerium. Flavonoids account for approximately two thirds of the phenolics in our diet as well as a high percentage of the secondary metabolites in Nerium oleander (14). There is also a report on antioxidant activity of essential oil of Nerium oleander flower (15).
However, owing to the differing antioxidant potential of compounds with different polarities in complex samples, all methods for assessing the antioxidant capacity of these samples are strongly affected by solvents extraction. The purpose of this study was to determine the total phenolic content and the antioxidant activity of water, methanol, water : methanol and acetone extracts of the leaves and flowers of Nerium oleander by employing various in-vitro methods including the reducing power, total antioxidant capacity and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity.
Experimental
Chemicals
All chemicals and solvents used were of analytical grade. Folin-Ciocalteu reagent, 1,1-diphenyl-2-picrylhydrazyl (DPPH), potassium ferricyanide, trichloroacetic acid, ferric chloride, Butylated hydroxyanisole (BHA), ascorbic acid, gallic acid, sodium phosphate dibasic, and sulfuric acid were purchased either by Sigma chemical or Merck Co. The aerial parts of Nerium oleander were collected from Babolsar in September 2010 and identified by Dr. A. Naghinezhad. A voucher specimen is deposited in the herbarium of the University of Mazandaran (Nr. 1500).
Extraction procedures
The extraction from dried and finely powdered leaves and flowers of Nerium oleander were carried out using four different solvents to compare the effect of extraction solvents on the total phenolic content, reducing power, radical scavenging and antioxidant activity. These solvents included methanol, methanol : water (50 : 50, v/v), water and acetone. The plant sample (1 g) was extracted with 3 × 20 mL of solvent under stirring at room temperature, till the solvent became colorless. Each extract was centrifuged (3500 rpm; 5 min.) and filtered through Whatman No. 1 filter paper. The filtrates were evaporated to dryness in vacuo at 40°C in a rotavapor. The dried samples of each extract were weighed to determine the yield of soluble constituents and stored at 4°C until use.
Determination of total phenolic content
The total phenolic content of each extract was assessed approximately by using the Folin-Ciocalteu phenol reagent method (16). Briefly, 20 μL of each extract solution was mixed with 1.58 mL distilled water and 100 μL of Folin-Ciocalteu reagent, followed by the addition of 300 μL of Na2CO3 solution (7%) after 1 min. Subsequently, the mixture was incubated by shaking at room temperature for 2 h. Then, its absorbance was measured at 760 nm. Gallic acid was used as a standard for calibration curve. The phenolic content was expressed as gallic acid equivalents in milligrams per gram of the dried material using the following linear equation based on the calibration curve:
A = 0.0468 C + 0.0006
R2 = 0.9968
Where A stands for the absorbance and C is the concentration as gallic acid equivalents (μg/mL).
Reducing power assay
The reductive potential of the extracts was determined according to the method prescribed by Oyaizu (17). Briefly, the different concentrations of the extracts, the standard compounds, ascorbic acid and gallic acid, (5-100 μg) in 1 mL of distilled water were mixed with 2.5 mL of phosphate buffer (0.2 M, pH = 6.6) and 2.5 mL of potassium ferricyanide [K3Fe (CN)6] (1% w/v). The reaction mixtures were incubated at 50°C for 20 min. A portion (2.5 mL) of trichloroacetic acid (10% w/v) was added to the mixtures. A volume of 2.5 mL of solutions were mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 (0.1% w/v). The absorbance of all the samples solutions were measured at 700 nm. An increase in the absorbance of the reaction mixture indicated greater reducing power. DPPH radical scavenging activity
The ability of the extracts to scavenge the DPPH radicals was determined according to the method of Blois (18). Briefly, 1 mL of methanolic solution of DPPH˙ (0.1 mM) was mixed with 3 mL of extract solution in methanol (containing 2-25 μg of the dried extract). The mixture was then vortexed vigorously and allowed to stand at room temperature for 15 min. The absorbance was measured at 515 nm and its activity was expressed as the percentage of DPPH˙ scavenging relative to the control sample using the following equation:
100 ×DPPH scavenging activiyy (%)=
Absorbance of control - Absorbance of sampleAbsorbance of control
Total antioxidant capacity
This assay is based on the reduction of Mo (VI) to Mo (V) by the sample and the subsequent formation of a green phosphate/Mo (V) complex at acidic pH (19). An aliquot of 0.3 mL of the sample solution (containing 100 μg of the dried extract in the corresponding solvent) was combined in a glass tube with 3 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). The tubes were incubated in a boiling water bath at 95°C for 90 min. Then, the samples were cooled to room temperature and the absorbance of the resultant solution was measured at 695 nm against the blank. A typical blank solution was prepared in the same conditions by replacing the sample with an appropriate volume of the same solvent used for the sample. Statistical analysis
All the experiments were carried out in triplicates and the results are reported as mean ± standard deviation. The analysis was performed using GraphPad Prism for windows.
Results and Discussion
Extract yield and total phenolics
The yields and amounts of the total phenolic contents for different extracts from the leaves and flowers of Nerium oleander are listed in Table 1.
| Leaves extracts | Yield (%) | Total phenolics (%) | Flowers extracts | Yield (%) | Total phenolics (%) |
|---|---|---|---|---|---|
| LM | 18.7 | 4.54 ± 0.23 | FM | 48.8 | 7.52 ± 0.93 |
| LMW | 22.1 | 4.25 ± 0.23 | FMW | 41.4 | 7.15 ± 0.43 |
| LA | 12.6 | 2.08 ± 0.38 | FA | 13.7 | 6.24 ± 0.57 |
| LW | 11.6 | 4.21 ± 0.29 | FW | 35.1 | 7.13 ± 0.49 |
The amount of the total phenolic content expressed as mg gallic acid equivalent per g of dried material ranged from 2.628 in the acetone extract to 9.402 in the aqueous methanol extract and 8.547 in the acetone extract to 36.690 in the methanol extract for the leaves and flowers respectively. Aqueous methanol and methanol were found to be the most effective solvents in the extraction of the phenolic content from Nerium oleander leaves and flowers, respectively. This is in agreement with the reports in literature that methanol is a widely used and effective solvent for extraction of phenols.
Reducing power
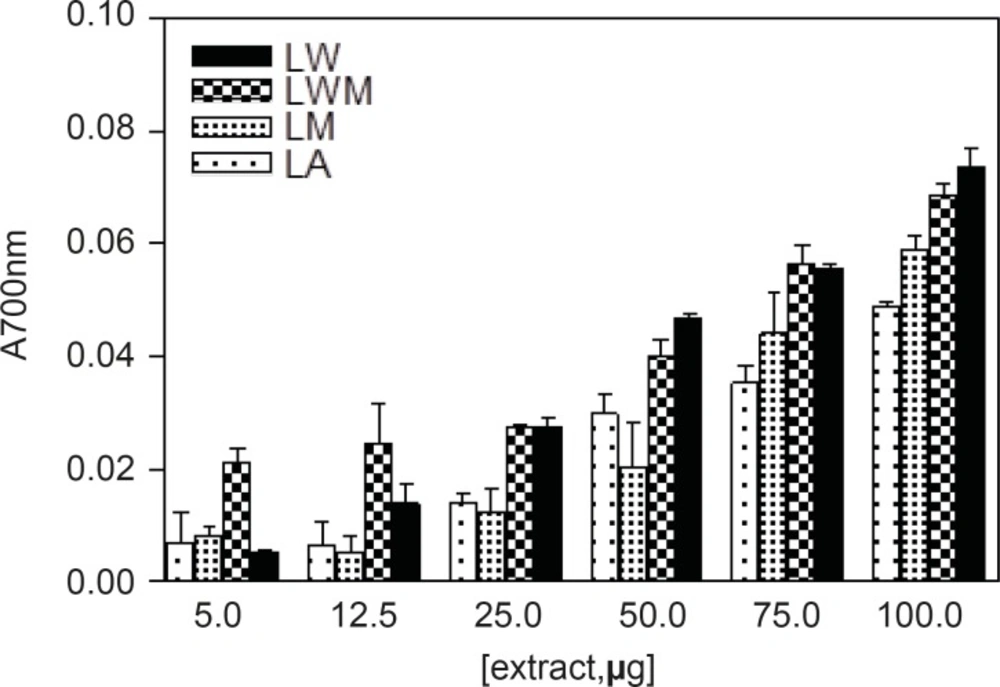
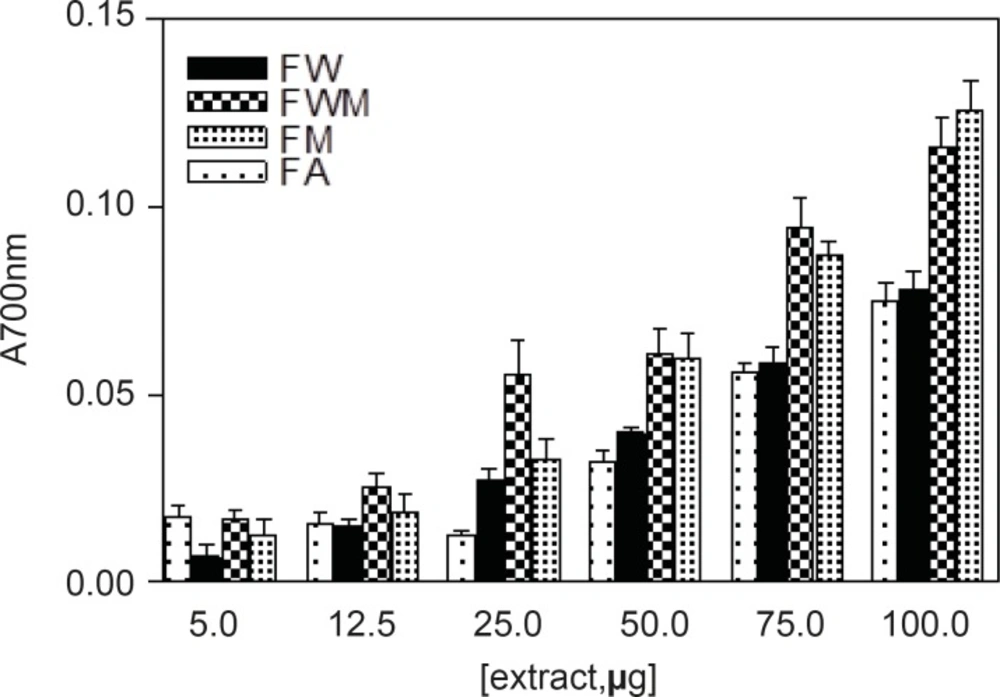
Different studies have indicated that the reducing capacity of bioactive compounds is associated with the antioxidant activity (20). In this study, the reducing power of Nerium oleander extracts and the reference compounds (ascorbic acid and gallic acid) were determined. All extracts showed some degree of electron donation capacity in a concentration-dependent manner (Figures 1 and 2), but the capacities were lower than that of ascorbic acid or gallic acid. The methanolic and aqueous methanolic extracts of the leaves containing the highest amount of total phenolics, were the most potent reducing agents, whereas the acetone extract containing that showed the least amount of phenolics content, was the weakest in the activity.
Reducing power
Different studies have indicated that the reducing capacity of bioactive compounds is associated with the antioxidant activity (20). In this study, the reducing power of Nerium oleander extracts and the reference compounds (ascorbic acid and gallic acid) were determined. All extracts showed some degree of electron donation capacity in a concentration-dependent manner (Figures 1 and 2), but the capacities were lower than that of ascorbic acid or gallic acid. The methanolic and aqueous methanolic extracts of the leaves containing the highest amount of total phenolics, were the most potent reducing agents, whereas the acetone extract containing that showed the least amount of total phenolics, were the most potent reducing agents, whereas the acetone extract containing that showed the least amount of phenolics content, was the weakest in the activity.
Reducing power
Different studies have indicated that the reducing capacity of bioactive compounds is associated with the antioxidant activity (20). In this study, the reducing power of Nerium oleander extracts and the reference compounds (ascorbic acid and gallic acid) were determined. All extracts showed some degree of electron donation capacity in a concentration-dependent manner (Figures 1 and 2), but the capacities were lower than that of ascorbic acid or gallic acid. The methanolic and aqueous methanolic extracts of the leaves containing the highest amount of total phenolics, were the most potent reducing agents, whereas the acetone extract containing that showed the least amount of phenolics content, was the weakest in the activity.
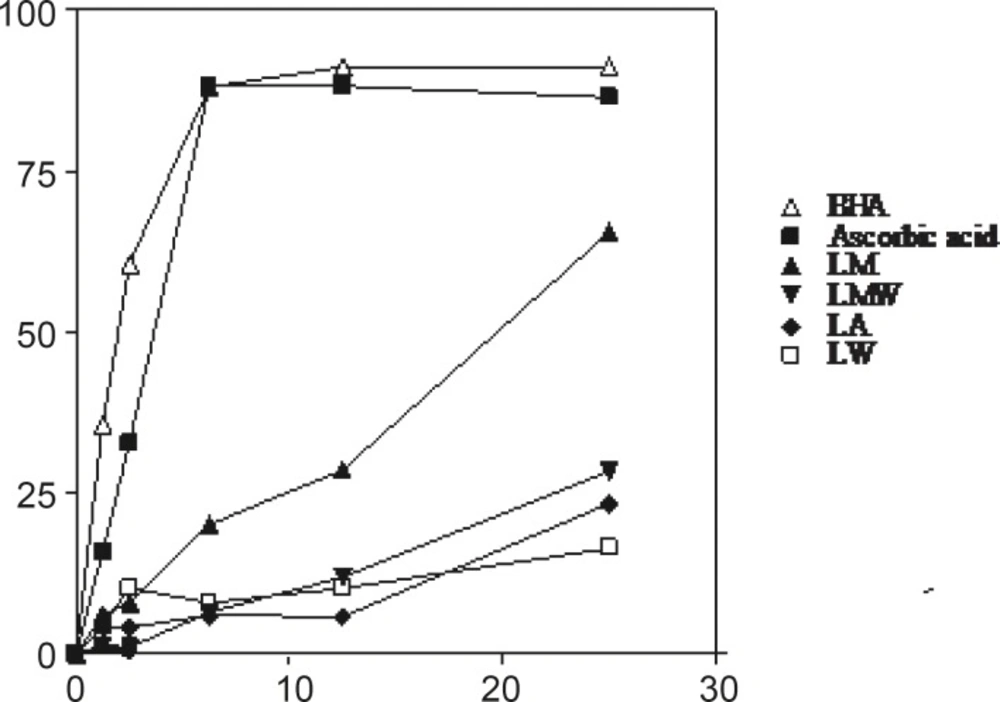
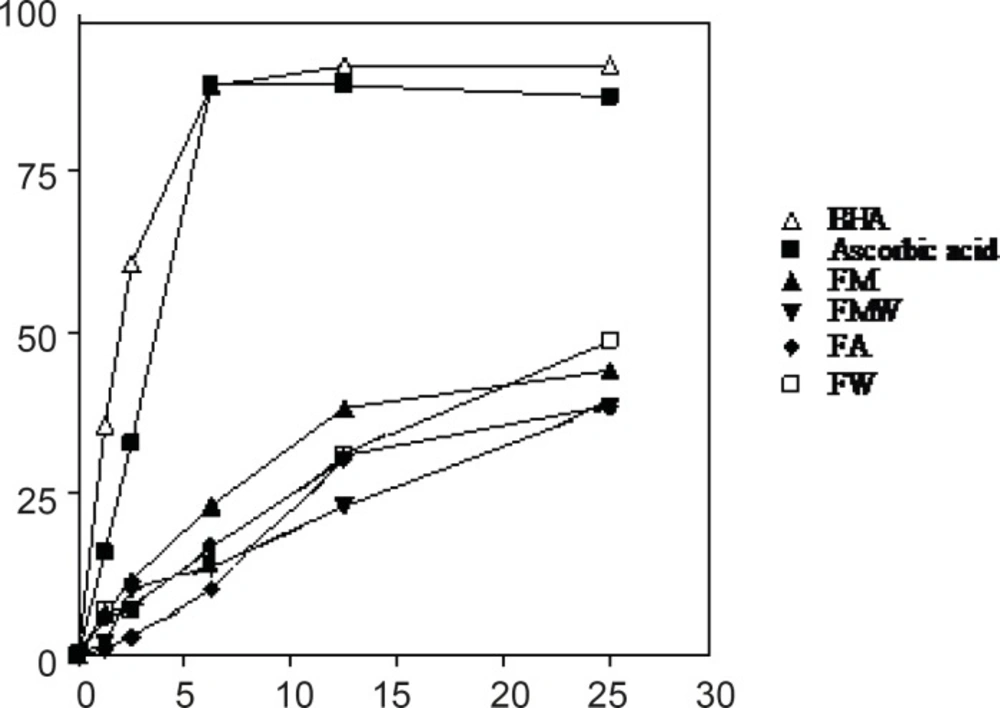
DPPH radical scavenging activity
The DPPH radical has been extensively used to evaluate the reducing substances (21) and is a useful reagent for investigating the free radical scavenging activities of compounds. The radical scavenging activity of four extracts from Nerium oleander leaves and flowers, ascorbic acid and BHA at five concentrations was tested by DPPH method and the results are illustrated in Figures 3 and 4. The methanolic extracts from the leaves and flowers that contained the highest amounts of the total phenolics, were found to be the most active radical scavengers followed by aqueous methanol and acetone extracts. Interestingly, the extracts from the leaves showed relatively high DPPH scavenging activity comparing with those extracts from the flowers of Nerium oleander.
| Leaves extracts | Reducing Power (100 μg extract in test) | Flowers extracts | Reducing Power (100 μg extract in test) |
|---|---|---|---|
| LM | 0.059 ± 0.004 | FM | 0.126 ± 0.014 |
| LMW | 0.068 ± 0.004 | FMW | 0.116 ± 0.013 |
| LA | 0.049 ± 0.001 | FA | 0.075± 0.008 |
| LW | 0.074 ± 0.005 | FW | 0.078 ± 0.008 |
Total antioxidant capacity
Using the phosphomolybdenum assay, which is a quantitative method to evaluate the water-soluble and fat-soluble antioxidant capacity (total antioxidant capacity), shows the four extracts exhibited different levels of activity in a concentration-dependent manner. A higher activity was observed for 100 μg extract in the methanolic leaves extract (1.281 mg of ascorbic acid equivalents/mg extract) and in the aqueous extract of the flowers (2.930 mg of ascorbic acid equivalents/mg extract) (Table 3).
| Sample | Antioxidant activitya† | Sample | Antioxidant activity |
|---|---|---|---|
| LM | 1.280 ± 0.02 | FM | 2.330 ± 0.04 |
| LMW | 1.246 ± 0.01 | FMW | 1.386 ± 0.02 |
| LA | 0.982 ± 0.01 | FA | 1.596 ± 0.04 |
| LW | 0.912 ± 0.004 | FW | 2.930 ± 0.01 |
| BHA | 6.474 ± 0.05 | BHA | 6.474 ± 0.05 |
In conclusion, we showed that Nerium oleander possesses an effective antioxidant activity, which includes free radical scavenging and reducing power. Various solvent extracts from Nerium oleander leaves and flowers showed different levels of antioxidant activity in different test systems in a concentration- dependent manner. The antioxidant activity was correlated with the amount of the total phenolic content present in the respective extracts in each assay. Methanol and aqueous methanol were proved to be the most efficient solvents for the extraction of antioxidants from Nerium oleander flowers and leaves. The related extract contains the highest amount of phenolic contents and also exhibited the strongest antioxidant capacity in all the assays used here. These findings are useful for further research to identify, isolate and characterize the specific compound which is responsible for the higher antioxidant activity.