Introduction
Epilepsy is one of the most prevalent neurological disorders which affects about 0.5- 1 percent of the world’s population (1). Epilepsy is resulted from a recurrent spontaneously abnormal electrical discharge of a group of neurons in the brain and exhibits as seizure occurrence in the patients (2). Glutamate and γ-aminobutyric acid (GABA) are two important excitatory and inhibitory neurotransmitters in epilepsy (3, 4). In spite of the generally acceptable treatment of epilepsy by anticonvulsant drugs, about one-third of this population suffers from un-prevented neurological changes induced by epileptic seizures and also exhibit some accompanied side effects. The long time seizure-induced neuronal activity might result in neurological changes and finally is ended by neuronal death (5). Oxidative stress and free radicals production are of the most important mechanisms by which neurological disorders such as epileptic seizure occur (6, 7). Nitric oxide (NO) is known as a neurotransmitter in the brain that has shown paradoxical role in seizure modulation, as an inhibitor (8-10) and promoter (11, 12) in different cases. The final product of lipid peroxidation is malondialdehyde (MDA), while MDA level could be considered as an index of lipid peroxidation. Increased level of MDA, as an index of lipid peroxidation in the PTZ mice may lead us to the conclusion that free fatty acids and free radicals are made from membrane phospholipid metabolism. Superoxide dismutase (SOD) is an intracellular antioxidant enzyme that catalyses converting the peroxidase to hydrogen peroxide (H2O2) in order to protect the cell from superoxide radicals and oxidative stress.
Black mustard is a many-branched, aromatic, weedy annual plant, growing up to 4 meters in height. It has showy, smallish yellow flowers. It belongs to the botanical family of Brassicaceae. Its seeds grow in long, slender pods. Each pod contains 10-12 brown or black seeds. Mustard seeds contain omega-3 fatty acids, essential oils, the minerals selenium, phosphorus, manganese, magnesium, iron, calcium, zinc, vitamins A, B-complex, and C, dietary fibre, protein, and phytonutrients (13). In Iranian traditional medicine, Brassica nigra seed has been used as a sedate for neurotic pain and rheumatoid arthritis, treatment of the brain and lung edema, paralysis, migraine and epilepsy (14). Experimental reports have shown the antioxidant (15), hypoglycemic (16), anticancer (17) and antimicrobial effect (18) of Brassica nigra seed. There are evidences implying the anti-epileptic effect of Brassica nigra in Iranian traditional medicine (14). Furthermore, the effect of this plant on oxidative stress and free radicals production is also reported (19). Therefore, in the present study we have investigated the antiepileptic effect of brassica nigra seed extract via assessing the anticonvulsant property of the plant. We have also tried to consider the antioxidant effect of the plant using MDA, NO and SOD assessment using the kindling method. We have also compared the anticonvulsant and antioxidant effects of Brassica nigra with valproate as a standard drug for epilepsy.
Experimental
Animals
In this experimental research, a total of 60 male Albino mice weighing 20-25 g (Razi Institute, Iran) were randomly divided into six experimental groups including: 1- control, 2- PTZ-induced kindled, 3- positive control group which beside the PTZ received valproate 100 mg /Kg (IP (Sigma, USA)) as an anti-convulsant drug, 4, 5 and 6- treatment groups which beside the PTZ received the brassica nigra seed extract in three doses of 75, 150 and 300 mg/Kg; IP. Ten mice were housed in each cage at temperature of 21 ± 2ºC and 12 h light-dark cycling. The mice had free access to standard food and tap water ad libitum. The experimental protocol was approved by the Ethic Committee of the University.
Kindling
All animals except the control groups (group 1) were kindled by a total of 11 period injections of PTZ (35 mg/Kg; IP). PTZ (Sigma) was dissolved in sterile isotonic saline. Each administration was carried out every second day and in a period of 22 days. Mice were observed for 30 min after the last drug administration. After an additional 30 min, the mice were observed for lethality before returning to the home cage. The challenge dose of 75 mg/Kg PTZ was injected to the kindled mice on 26th day (the test day), which could produce convulsions (tonic-clonic) and lethality (5). In the four treatment groups (valproate and different doses of Brassica nigra), PTZ was administrated 30 min after the first treatment with valproate and different doses of Brassica nigra. However, the exhibited phases of seizure (0-6) were observed and categorized using the following scale (19) for 30 min after the PTZ injection. The scale introduces six phases as follows; 0: No response, 1: Ear and facial twitching, 2: Convulsive waves axially through the body, 3: Myoclonic body jerks, 4: Generalized clonic convulsions turn over into side position, 5: Generalized convulsions with tonic extension episode and status epilepticus, 6: Mortality.
Preparation of plant hydro-alcoholic extract
The medicinal plant of Brassica nigra seed was provided from the local market and was scientifically identified by the department of Botany of Shahed University. To prepare the hydro-alcoholic extract, using percolation method, 50 g of cleaned Brassica nigra seed was crushed and mixed at ratio of 1 to 5 with ethanol 80% and kept for 24 h at room temperature. During this time, it was stirred several times. Then, the solution was transferred to a percolator and then the deposit was separated using paper filter. The tab of the percolator released 2-3 drops of the solution per minute. After filtration, it was maintained in a water bath at 40°C for 16 h to let the alcohol be evaporated from the filtered solution to reach a final concentration of 25% (20).
Sample preparation and biochemical assays
After the injection of the challenge dose of PTZ and behavioral analysis, mice were decapitated. The brains were removed quickly and were washed in cold saline for two times. They were placed in freezer (-30°C), in a glass bottle (less than 10 h). Then, the brain pieces (cutting the brain tissue using the scissors) were homogenized using four times ice-cold Tris-Hcl buffer (50 mM, pH = 7.4) for two min at 5000 rpm. MDA and NO levels were measured at this phase. The homogenized solution was then centrifuged for 60 min at 5000×g to remove debris. The supernatant solution was then extracted with a mixture of ethanol : chloroform (a volume with ratio of 5 : 3). After centrifugation at 5000×g for 30 min, the clear upper layer (the ethanol phase) was taken and used for the evaluation of the SOD activity. All experiments were carried out at +4°C (5).
MDA evaluation
The MDA concentration (thiobarbituric acid reactive substances, TBARS) in the supernatant was measured according to the following protocol. Briefly, trichloroacetic acid and TBARS reagent were added to the supernatant, then mixed and incubated at 100˚C for 80 min. After being cooled on ice, samples were centrifuged at 1000×g for 20 min and the absorbance of the supernatant was read at 532 nm (21).
NO evaluation
Supernatant NO content was assayed by the Griess method. Since NO is a compound with a short half-life and is rapidly converted to the stable end products of nitrate (NO3-) and nitrite (NO2-), the principle of the assay is the conversion of nitrate into nitrite by cadmium and followed by color development with Griess reagent (sulfanilamide and n-naphthyl ethylenediamine) in acidic medium (22). The total nitrite was measured by Griess reaction. The absorbance was determined at 540 nm with a spectrophotometer (5).
SOD activity evaluation
SOD activity measurement was according to the following protocol. Briefly, supernatant was incubated with xanthine and xanthine oxidase in potassium phosphate buffer (pH = 7.8, 37°C) for 40 min and NBT was added. Blue formazan was then monitored spectrophotometrically at 550 nm. The amount of protein that inhibited the NBT reduction to 50% of maximum was defined as 1 nitrite unit (NU) of SOD activity (23).
Statistical analysis
Data were expressed as mean ± SEM. Comparison of seizure phase in different injection periods was carried out using repeated measurement of two-way analysis of variance (ANOVA). Other data were subjected to one-way ANOVA and its related post-hoc tests. P-values less than 0.05 were considered as significant differences.
Results and Discussion
Effect of Brassica nigra on the PTZ-induced kindling intensity
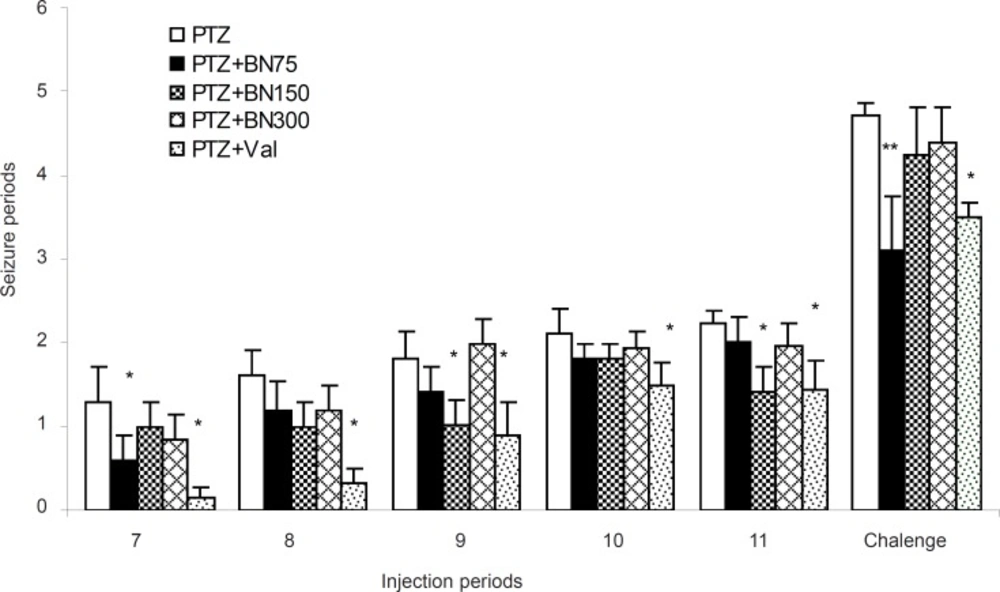
Statistical analysis of results indicates that there are no significant differences among the experimental groups in seizure intensity until the 7th injection (data is not shown). Moreover, as it is shown in Figure 1, hydro-alcoholic extract of Brassica nigra with 75 mg/Kg dose at 7th injection and with 150 mg/Kg at 9th and 11th injection were able to reduce the PTZ-induced seizure significantly [F (14, 2) = 4.63, p < 0.018]. However, valproate (100 mg/Kg) has reduced seizure intensity in all periods significantly. At the 12th injection, (challenge dose), Brassica nigra 75 mg/Kg and valproate, had similar reducing effect on seizure intensity without any significant difference.
Effect of Brassica nigra on the PTZ-induced kindling factors
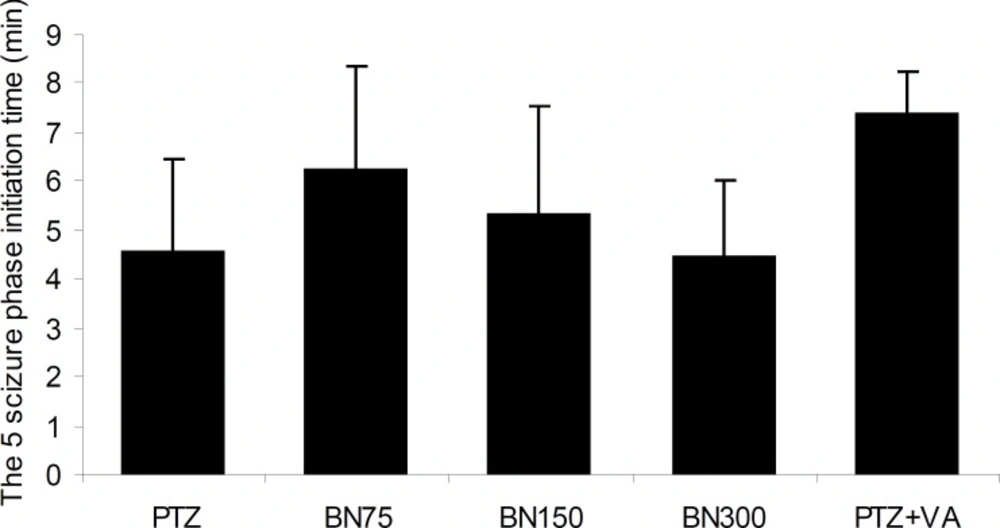
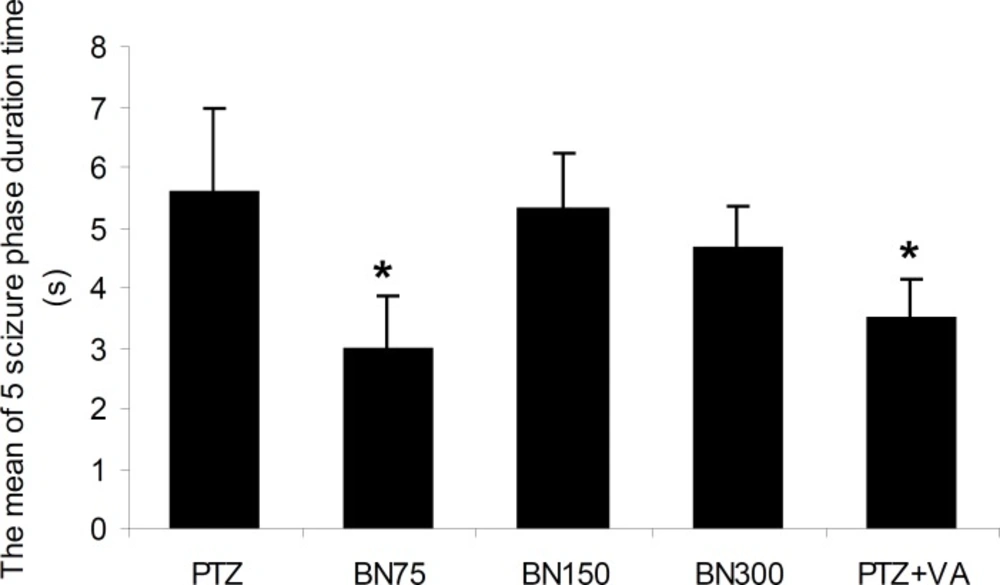
As could be seen in Figure 2, pretreatment of animals with different doses of Brassica nigra do not have any significant effect on the duration time that the mice reach to phase 5 seizures. In addition, Figure 3 indicates that only pretreatments of mice with Brassica nigra 75 mg/Kg and valproate 100 mg/Kg are able to reduce the period that mice remain in phase 5 of seizure significantly [F (4, 45) = 1.44, p < 0.02].
Effect of Brassica nigra on the biochemical indexes of oxidative stress and antioxidant
Table 1 indicates the brain levels of biochemical factor changes that are usually the indexes of oxidative stress in tissues, in kindled and non-kindled groups with or without pretreatment with valproate and brassica nigra extract. PTZ-induced kindling has significantly increased the MDA level in the brain tissue of kindled mice compared to the control group [F (4, 54) = 4.66, p < 0.001]. However, the significant reductive effect of PTZ on the SOD level in the brain as compared to control mice was also observed. Nonetheless, the NO level in the brain of kindled mice compared to the control group was unchanged.
| Enzyme | Micromole/g protein NO | nmol/g protein MDA | U/mg protein SOD |
|---|---|---|---|
| Groups | |||
| Control | 0.03 0.53 ± | 1.89 17.69 ± | 0.13 ± 00 |
| PTZ | 0.6 ± 0.03 | 25.63 ± 2.11 * | 0.1 ± 0.01* |
| PTZ + valproate | 0.4 ± 0.04*# | 20.46 ± 1.98 | 0.13 ± 0.03 |
| PTZ + BN (75 mg/Kg) | 0.66 ± 0.07 | 23.61 ± 1.23 | 0.12 ± 00 |
| PTZ + BN (150 mg/Kg) | 0.74 ± 0.03*# | 26.21 ± 1.64 | 0.16 ± 0.01*# |
| PTZ + BN (300 mg/Kg) | 0.81 ± 0.06 *# | 18.29 ± 1.18 # | 0.14 ± 0.02 |
Valproate administration was only able to significantly decrease the brain’s NO activity in the PTZ-kindled mice compared to the control and non-treated PTZ-kindled mice [F (4, 45) = 10.21, 0.001] and did not change MDA and SOD level in the brain tissue. Interestingly, in the pretreated group with 150 and 300 mg/ Kg doses of brassica nigra, the NO brain content had a significant increase compared to the other groups. The MDA level of the brain tissue, only in brassica nigra treated group (300 mg/Kg) compared to the PTZ-kindled mice, has been significantly decreased [F (4, 45) = 3.19, p < 0.001]. Finally, the effective dose of brassica nigra on the lowering of SOD level of the brain compared to the control and PTZ-kindled mice was 150 mg/Kg.
Brassica nigra seed has been used as a treatment for epilepsy in Iranian traditional medicine (13). In the present research, it is observed that Brassica nigra seed extract could reduce the intensity, improvement and duration time of PTZ-induced seizure. Our data analysis showed that the hydro-alcoholic Brassica nigra extract in lower dose could significantly reduce the duration time that mice remain in phase 5 of seizure. It indicates that our results are in consistence with previous reports. Experimental researches suggest the existence of flavonoids with antioxidant effects in the hydro-alcoholic Brassica nigra seed (6). The seed also consists of vitamin A that is a potent antioxidant. So, vitamin A is able to prevent the kindling and convulsion, and is also able to inhibit the challenge of dose-induced tonic seizure (24), one of the mechanism’s actions of the plant that could be related to it.
Free radicals are involved in pathogenesis of many diseases such as epilepsy. The important effect of free radicals is membrane lipid peroxidation and tissue injury by which results in cell membrane destruction and its dysfunction. Normally, biological effects of free radicals in the body is controlled by a lot of antioxidants such as vitamins A, C and E, glutathione and also via anti-oxidant enzymes like glutathione reductase (GR), glutathione peroxidase (GP), SOD and catalase (25, 26). Generalized epilepsy is accompanied by reversible convulsing and can induce some species of reactive oxygen and superoxide in the brain (27, 28). Since it is supposed that free radicals mediate the convulsion improvement, nowadays, searching for antiepileptic drugs with antioxidant and neuroprotective effects are of interests. However, some scientists suggest that only NMDA receptor activation and NO production, without glutamine synthetase inhibition are involved in the seizure (29).
Frantseva et al., using mice with amygdale convulsion, have shown the production of oxygen radicals following the seizure, and probably these radicals are involved in the seizure improvement and convulsion-induced neuronal death. They have also shown that seizure is able to increase lipid peroxidation in both hemispheres and cell death in all areas of hippocampus. Interestingly, they have observed that during the seizure, antioxidants inhibit the lipid peroxidation in both hemispheres and cell death in the hippocampus (30).
It has been observed that antioxidants inhibit the PTZ-induced seizure significantly and reduce the seizure-induced oxidative stress (5). Furthermore, in epileptic patients, the serum level of antioxidants is reduced and lipid peroxidation is increased and both effects are correctable with antiepileptic drugs (26). In addition, possibly PTZ is a starter of various processes such as membrane phosphorylation, proteolysis, and nuclease and consequently release of free fatty acids, diasylglycerols, eicosanoids, lipid peroxides and free radicals (31). In the present study, significant increase of MDA as an index of lipid peroxidation and meaningful reduction of antioxidant enzyme (SOD) in the PTZ-induced kindled group lead to the production of free radicals and existence of oxidative stress in the brain of the kindled mice. Therefore, this research is in accordance with the theory that in the PTZ-induced animals, the oxidative stress is possibly one of the parameters that participate in the pathophysiology of epilepsy. In the present study, 300 mg/Kg of Brassica nigra could decrease the MDA level compared to the PTZ mice, in a way that one can conclude probably the antioxidant effect of Brassica nigra was able to decrease the oxidative injury, lipid peroxidation and MDA reduction. Possibly, the reductive effect of the brassica nigra extract on the seizure was resulted from the antioxidant property of the plant.
SOD is from antioxidant enzymes and catalyzes the conversion of superoxide to hydrogen peroxide and in this way, protects the cell against the superoxide and consequent oxidative stress. In the present study, it is observed that Brassica nigra 150 mg/Kg could increase the SOD level compared to the PTZ mice. This result can lead to the conclusion that probably Brassica nigra seed with antioxidant effect and deletion of free radicals is able to preserve the antioxidant enzyme SOD and consequently affect seizure intensity and duration.
Nowadays, NO is known as an important neurotransmitter that in addition to various physiological duties, is also related to synaptic plasticity, neuronal excitability regulation, and epileptic activity (8, 32). Controversial effects of NO have been obtained on the PTZ-induced convulsion. Oliveria et al. in 1997 have shown that NOS inhibition in kindling model amplifies the 60 mg/Kg PTZ-induced seizure intensity, but has protective effect against 80 mg/Kg PTZ-induced tonic seizures (33). So, they have concluded that the proconvulsant or anticonvulsant activity of NOS and NO inhibitors is dependent on the PTZ dose and the seizure model. Researchers have attributed the protective and inhibitory effect of NOS on high doses of PTZ to the contribution of NO in the proconvulsant effect of limbic system (33, 34). Using nNOS mice (lacking nNOS gene) and nNOS (neuronal NO synthetase) inhibitors, they have concluded that basic and enhanced levels, implies negative and positive modulatory effects respectively (34). It is suggested that the anticonvulsant role of NO is related to an implied feedback of NO on the NMDA receptor activation via different mechanisms (8). However, NO is known as a molecule that can easily react with O2.- radicals in the brain and reduce the oxidative stress-induced damage by eliminating free radicals (25). Controversial results make difficulties in predicting pro or anti-convulsant effect of NO molecule. Anyway, in the present research, the NO level is decreased in PTZ group compared to the control and is increased significantly in brassica nigra-treated group (300 mg/Kg) compared to the PTZ mice. This indicates that probably brassica nigra seed extract can have suppressing effect on seizures via NO synthesis mechanism activation. Probably, the reduced level of NO in PTZ mice is resulted from free radicals production at seizure time, and its consumption due to its cleaning effect. Besides, the enhanced level of NO in the group treated with brassica nigra seed extract is due to its antioxidant effect by which it eliminates O2-radicals, and consequently prevents lipid peroxidation and oxidative stress-induced injury that result in the NO level increment.
In the chemical kindling model with PTZ, which is identified by an increase in the seizure induction potential, GABA receptor is involved (36, 37). PTZ in single dose or repeated administration can affect GABAergic system. Furthermore, it has been reported that PTZ is able to block the flow of chloride ionophore complex to the GABA receptor (38). Flumazenil, as an antagonist of benzodiazepine binding site of GABAA receptor increased the GABA-dependent chloride uptake in cultured cortical neurons (39). Using the flutamide (androgen receptor antagonist) and flumazenil (benzodiazepine receptor) in PTZ-induced kindled mice, has shown that the PTZ action is applied via benzodiazepine receptor (40). Our results and these findings taken together, show that probably the anti-seizure effect of Brassica nigra is via GABAergic system and benzodiazepine receptor.
In conclusion, the present research indicates that hydro-alcoholic brassica nigra extract have anti-seizure effect on PTZ-induced kindling in mice. In addition, since the experimental epilepsy is mediated by oxidative stress and free radicals, it could be suggested that brassica nigra is able to prevent seizures by an antioxidant mechanism. However, the involvement of GABA receptor agonists in the Brassica nigra anti-seizure effect should not be ruled out.