Introduction
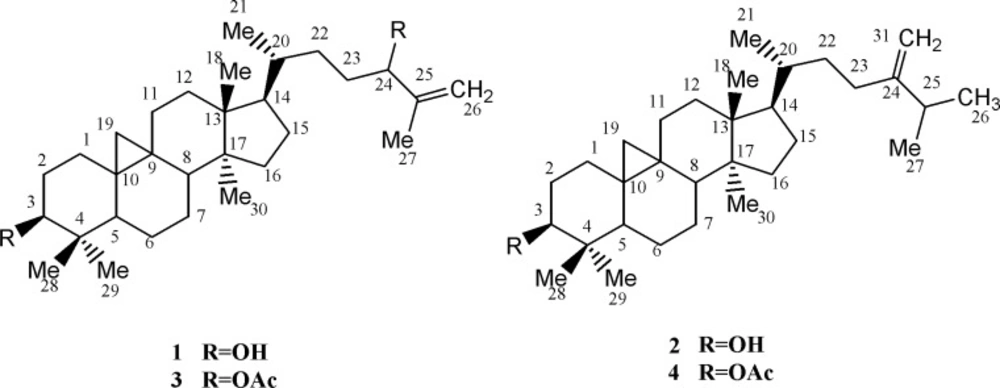
Cycloartanes (9, 19-Cyclolanostanes) are one of the main tetra-cyclic triterpene skeletons including the side chain and characteristic cyclopropane ring (1). They are the major key intermediates in the phytosterols biosynthesis (1). In addition, these compounds are used as specific chemotaxonomic markers in Euphorbia genus, which comprises well over 2000 species in tropical and temperate zones of asia and other parts of the world. In iran, 70 species are reported, 17 of which are endemic (2). Within our recent study on triterpenoids, present work describes the isolation and structure elucidation of two cycloartane type triterpenes from Euphorbia aellenii Rech. f. (Figure 1) along with their anti-proliferative activity on human peripheral lymphocytes and their structure-function studies on protein kinase C (PKC) that plays an important role, as an early event, in T-cell activation (3).
Experimental
General
Column chromatographie s (CC) were run silica gel 63-200 μm, LiChroprep® Si 60 (25-40 μm) and RP-18 (40-63 μm, Merck). The size exclusion chromatography (SEC) was rendered on sephadex (LH-20, Sigma-Aldrich) and recycling preparative HPLC on LC-908 (Hitachi Company, Japan), equipped with YMC Pack-Sil column (250*20 mm i.d). The NMR spectra were recorded on Bruker AV-300 (1H), AV- 600 (13C) and HR-ESI-MS on Waters Q-TOF Micro YA019 mass spectrometer.
Plant material
The aerial flowering parts of Euphorbia aellenii Rich. F. (euphorbiaceae) were collected from populations growing in Galil-e-Shirvan (Iran) and identified by Dr. Yasamin Naseh, herbaceous sciences research center at ferdowsi university of mashhad. A herbarium specimen No. 2024 is preserved in the herbarium of the faculty of pharmacy, isfahan university of medical sciences (Iran).
Isolation of cycloartanes
The air-dried powdered plant (7 Kg) was macerated for four days with MeOH (20 L×3), at room temperature. Filtration and in-vacuo evaporation resulted in a green gum (500 g), which was partitioned between methanol and n-hexane. The defatted methanolic extract was concentrated, dissolved in water, and extracted sequentially with chloroform, ethyl acetate, n-butanol, and water. The resulted fractions (Fr.1-Fr.4) were compared in-vitro for cytotoxic activity (4), showing LD50 value of 177.06 (96.90-255.61) and 770.66 (670.06 -916.40) μg mL− 1 for Fr.1 and Fr.2, respectively while other fractions (Fr.3 and Fr.4) were marked as non-cytotoxic. The most active fraction (Fr.1, 240 g) was subjected to silica gel column, using Hexane/CHCl3 (0→100), to render several fractions (Fr.1a-Fr.1f). Then, Fr.1b was eluted with Hexan/CHCl3 1:1 and chromatographed on silica gel (Hexane/EtOAc, 0→50) to come up with ten subfractions. Finally, 1 g of the fraction was eluted with Hexane/EtOAc 9:1, purified on recycling HPLC (Hexane/EtOAc 6:4, 4.0 mL/min), and consequently yielded two pure compounds: 1 (20 mg, tR 200 min) and 2 (10 mg, tR 220 min).
Preparation of cycloartane derivatives
A mixture of cycloartane type compound (10 mg), acetic anhydride (1 mL) and pyridine (0.5 mL) were stirred at room temperature for three days. The final point of reaction was controlled by TLC and the reaction mixture was poured over ice to decompose the remaining acetic anhydride. Thereafter, suspension was extracted twice with ethyl acetate (2:1 v/v), and evaporated in-vacuo to yield acetylated products (3 and 4), which was confirmed by Mass and H-NMR spectra (5).
Brine shrimp (artemia salina) cytotoxicity
Shrimp eggs were added to a specific tank containing artificial seawater, hatched within two days and transferred to sample vials (1000, 100 and 10 μg/mL). 24 h later, keeping vials under illumination, the surviving shrimps were counted to obtain LC50 and 95% confident intervals (4).
Proliferation assay
Peripheral human blood lymphocytes were incubated with different concentrations of the test compounds (0.5, 5, and 50 μg/mL (in triplicates) in supplemented RPMI-1640 along with phytohemagglutinin (PHA) at 37ºC in CO2 environment for 72 h. Further incubation for 18 h after the addition of thymidine [3H] (Amersham, UK) was done and cells were harvested using cell harvester (Innotech Dottikon, Switzerland). Finally, proliferation level was determined by the radioactivity count as CPM recorded from the Beta-scintillation counter (Beckman coulter, LS 6500, Fullerton, CA, USA) (6).
Docking methodology
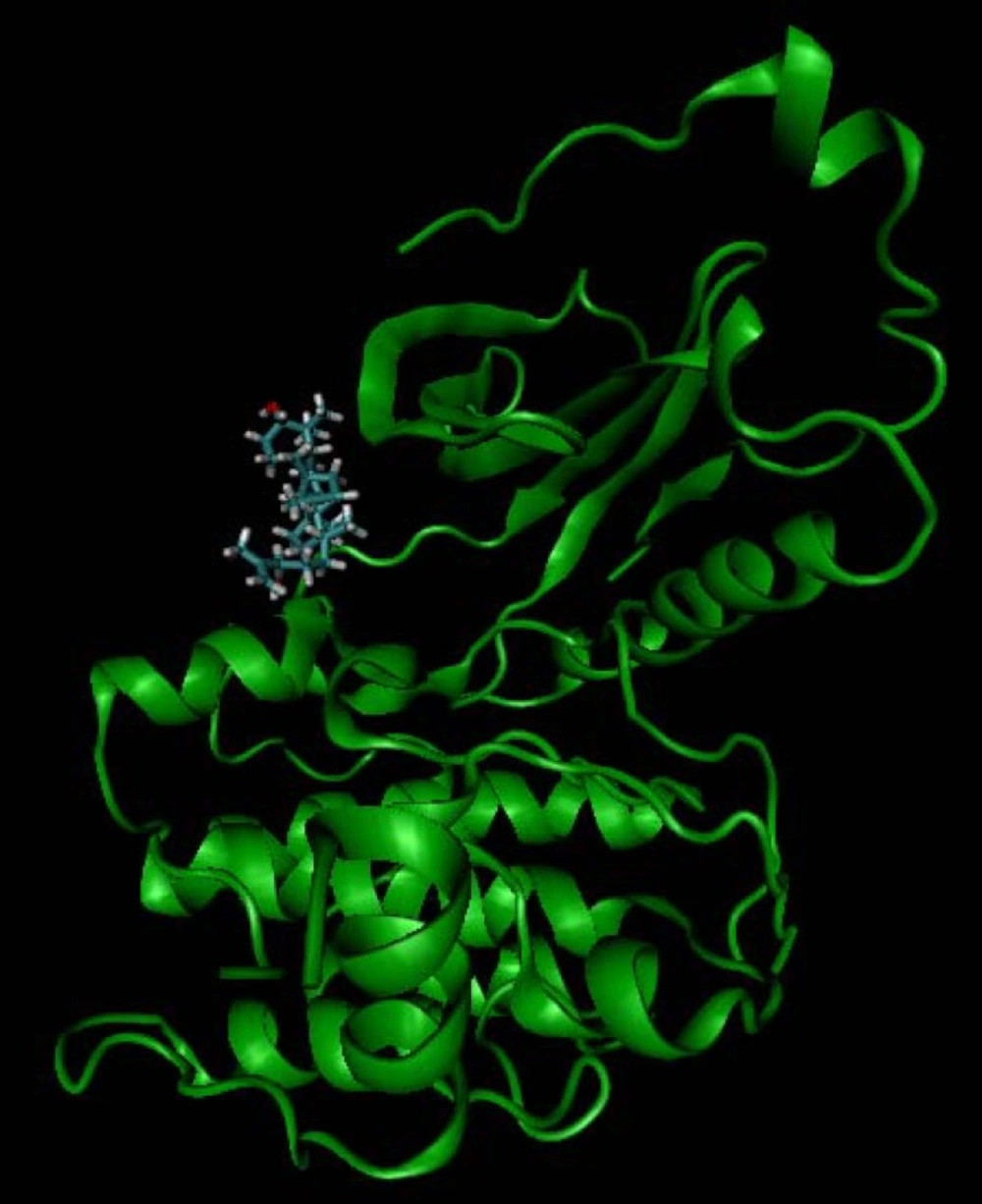
Using the crystal structure of protein kinase C (PKC), retrieved from the protein data bank (PDB code: 1zrz), compounds (1-4) were selected for docking process and optimized by Polak-Ribiere conjugate gradient algorithm and AM1 semi empirical method, implemented in HyperChem. The optimized structures were used as the input of auto dock tools and the partial charges of atoms were calculated using gasteiger-marsili procedure (7). Merging non-polar hydrogens, rotatable bonds were assigned; after removing the heteroatom including water molecules from protein, all missing hydrogens were added. Thereafter, determining Kollman united atom charges (8), non-polar hydrogens were merged to their corresponding carbons and as a final processing part in protein preparation, desolvation parameters were assigned to each atom. Using auto Grid tool, the grid maps (one for each atom type in the ligand, and one for electrostatic interactions) were constructed adequately large to include the active site of protein as well as significant regions of the surrounding surface. In all the cases, a grid map of 60 points in each Cartesian direction apart from a grid-point spacing of 0.375 A° (a quarter of the carbon-carbon single bond) were generated. By the ligand location in the complex, the maps were centered on the ligand’s binding site, searching favorable interactions with the functional groups. Based on lamarckian genetic algorithm (9), using the pseudo-solis and wets local search method (10), Auto Dock Tools were employed to produce both grid and docking parameter files i.e. .gpf and .dpf files. Applying 2.0 A° clustering tolerance to construct clusters of the closest compounds, the initial coordinates of the ligand were used as the reference structure. In addition, for the internal validation phase, ligand structure (corresponding HETATM and CONECT records) was extracted from the pdb file of CDK2 (2BTS). Later, assigning bond orders, missed hydrogens were added and a short minimization (100 steepest descent steps were taken, using MM+ force field with a gradient convergence value of 0.05 Kcal/mol A°) to release any internal strain. At last, docking results (PKC-ligand complexes) were visualized using VMD1.8.6 (11).
Statistical analysis
The IC50 values were calculated using Excel based program and reported as mean ± SD of the mean. Significance was attributed to p-values (p < 0.05) and the probability values obtained by the student t-test between the sample and control data.
Results and Discussion
Compound 1, white crystals with mp 180-184°C, showed the molecular formula of C30H50O2 based on positive EI-HR-MS m/z 442.3784 (calc. for C30H50O2: 442.3811, Δ 6.10 ppm) ), in accordance with the number and the multiplicity of 13C-NMR spectra (Table 1).
| 13C | 1 | 2 |
|---|---|---|
| 1 | 31.66t | 31.97t |
| 2 | 30.45t | 30.38t |
| 3 | 78.89d | 78.85d |
| 4 | 40.48s | 40.49s |
| 5 | 47.19d | 47.1d |
| 6 | 21.14t | 21.14t |
| 7 | 28.12t | 28.17t |
| 8 | 47.99d | 48.02d |
| 9 | 20.41s | 19.98d |
| 10 | 26.15s | 25.78s |
| 11 | 26.04s | 26.04t |
| 12 | 35.61t | 32.88t |
| 13 | 45.29s | 45.29s |
| 14 | 48.8s | 48.81s |
| 15 | 32.02t | 34.95t |
| 16 | 26.55t | 26.46t |
| 17 | 52.26d | 52.26d |
| 18 | 18.03q | 18.07q |
| 19 | 29.89t | 29.93t |
| 20 | 36.01d | 36.35d |
| 21 | 18.37q | 18.23q |
| 22 | 31.53t | 35.57t |
| 23 | 28.12d | 31.31t |
| 24 | 76.74d | 156.96s |
| 25 | 149.77s | 33.8s |
| 26 | 111.33t | 22.02t |
| 27 | 17.27q | 19.34q |
| 28 | 19.36q | 18.31q |
| 29 | 25.48q | 14.03q |
| 30 | 14.02q | 25.45q |
| 31 | - | 105.91t |
13C-NMR data for the cycloartanes (1 and 2).
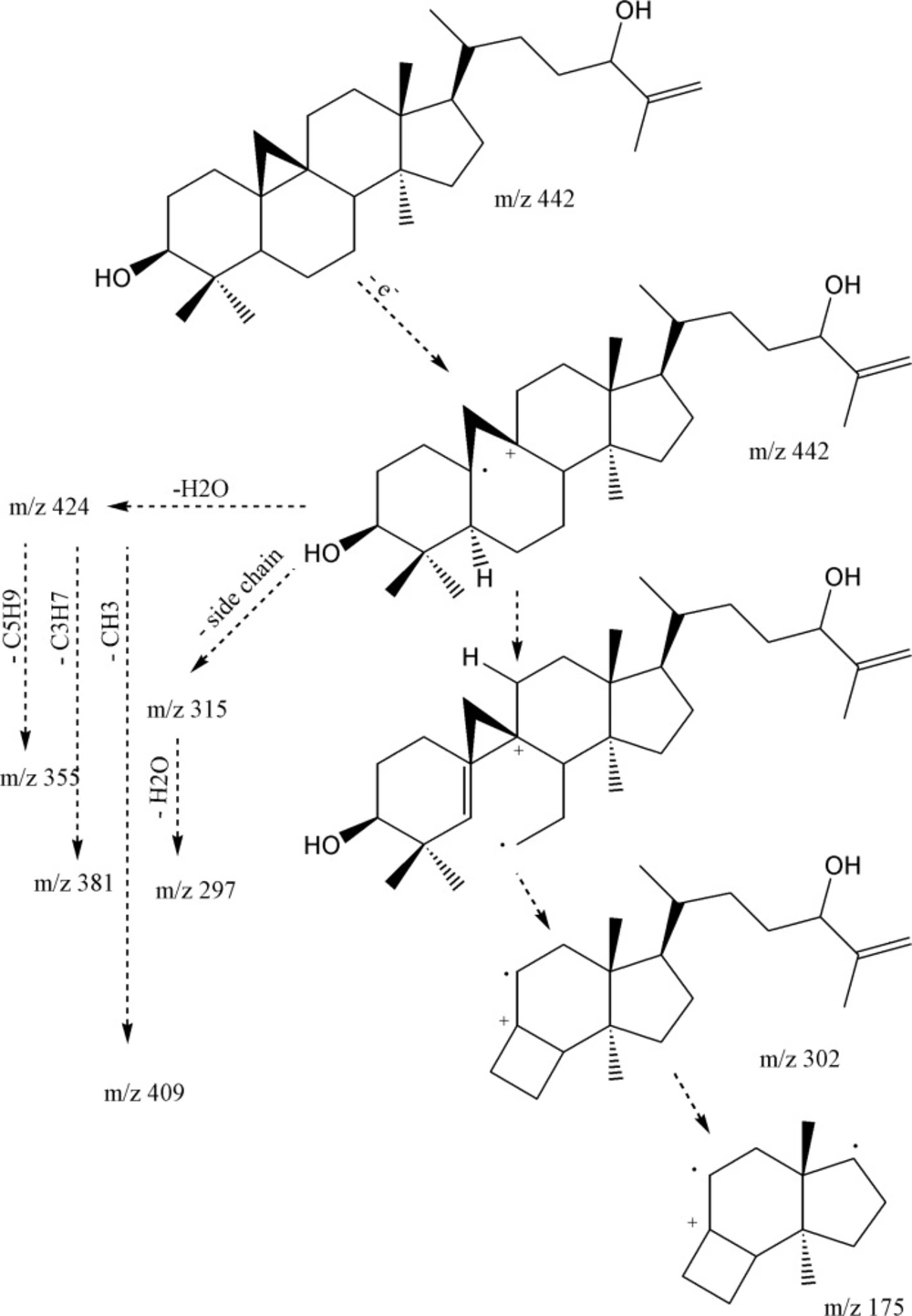
The IR spectrum confirmed presence of hydroxyl group (3373 cm-1), double bond absorption (1650 and 756 cm-1), C-O functions (1219, 1095 and 1026 cm-1), and cyclopropane C-H (3018 cm-1) together with C-H stretch bonds (2916 and 2848 cm-1). 1H-NMR revealed five singlet methyls at δH 1.70 (s, Me27), 0.94 (s, 6H: Me18, Me30), 0.87 (s, Me28) and 0.78 (s, Me29), one secondary methyl group at 0.84, and a pair of doublets in the up-field area (δH 0.30, J = 4.2 Hz and 0.52, J = 4.2 Hz), characteristic of cycloartane cyclopropane ring. A double doublet carbinolic proton at δH 3.25 (dd, Jax,ax = 11.1 , Jax,eq= 4.5 Hz, H3), with reference to its axial and α-orientation, assigned the hydroxyl group as 3β-OH. Using HMBCs, the downfield carbinolic proton at δH 3.99 (t, J = 6.3 Hz, H24), showed connectivity with a pair of olefinic protons at δH 4.80 (d, J = 1.2 Hz) and 4.90 br-s (each one H), suggesting a terminal methylene. As a whole, the six-degree of unsaturation and the 13C-NMR data (Table 1), suggested the presence of a double bond and, therefore, a pentacyclic skeleton. EI-MS fragmentation pattern, supported m/z 355.3018 [C25H39O]+ and 302.2616 [C21H34O]+, typical ions of 4,4’ dimethyl 9,19 cycloesterols (Figure 2).
The presence of the monounsaturated side chain was confirmed by the m/z 313.2502 [C22H33O]+, 315 [C22H35O]+ and 297.2587 [C22H33]+. In addition, 381.3153 [M-H2O-C3H7]+ together with 355.3018 [M-H2O-C5H9] +, fragmented due to the elimination of parts of side chain (during a Mc Lafferty process), inferred presence of hydroxyl group in side-chain as is shown in Figure 2 (11). Regarding to these findings and the published data (11), compound 1 was identified as cycloart-25-en-3β,24-diol.
HR-EI-MS identified compound 2, as C31H52O with molecular ion peak at m/z 440.4015 (calc. for C31H52O: 440.4018, Δ 0.7 ppm). The IR spectrum confirmed absorption of hydroxyl group (3311 cm-1), double bond peak (1640 and 771 cm-1), C-O functions (1219 cm-1) and C-H stretching at 3020 (cyclopropane ring), 2916 and 2848 cm-1. Six degree of unsaturation suggested a double bond (Table 1) and consequently five rings in the molecule. The resonances encompassed thirty-one carbons including seven methyls, twelve methylenes, six methines and six quaternary carbons. 1H-NMR revealed five singlet methyls at δH 1.01 (d, J = 3 Hz, Me27), 0.99 (d, J = 3 Hz, Me26), 0.94 (s, 6H: Me18, Me30 ), 0.88 (s, Me28), 0.86 (d, J = 6 Hz, Me21) and 0.79 (s, Me29), a pair of doublets in the up-field area at δH 0.30 and 0.53 (J = 4.25 Hz) indicative of cyclopropane ring characteristic of cycloartanes. A doublet of doublet proton at δH 3.26 (dd, Jax,ax = 11.0 , Jax,eq= 4.0 Hz, H3), indicative of equilateral β orientated hydroxyl-group, and one pair of olefinic protons δH 4.64 (d, J = 0.5 Hz) and 4.69 (br-s) related to exocyclic terminal methylene. Based on these data, compound 2 was determined as 24-methylene-cycloartan-3β-ol (12), confirmed by the EI-MS fragments m/z 425 [M-CH3], 407 [425-H2O], 315 [M-side chain], 297[315-H2O], 300[C22H36] and 175 [300-sidechain].
Compound 3 obtained by the acetylation of 1, was identified as 3β, 24-O-diacetyl-cycloart-25-en through EI-MS molecular ion peak m/z 526 [M]+, 466 [M-CH3COOH]+, 423[466-CH3CO], 406[466-CH3COOH]+ and 1H-NMR spectrum. The IR spectrum supported absorptions at 3020 (cyclopropane ring), 2936, 2868, 1736 (esteric carbonyl), 1650, 1456, 1373, 1244, 1026 and 758 cm-1 without hydroxyl group peak at [3500-3300 cm-1]. Likewise, the structure of compound 4 after acetylation of 2, was confirmed as 3β-O-acetyl-24-methylene-cycloartan on the bases of EI-MS molecular ion peak m/z 482 [M]+ and 422 [M-COOH]+, IR and NMR spectra. IR spectrum showed absorption at 3018 (Cyclopropane ring), 2916, 2848, 1736 (Esteric carbonyl), 1642, 1456, 1373, 1244, 1026 and 758 cm-1 without any peak at hydroxyl area [3600-3200 cm-1] and the signals of δH 4.64 (d, J = 0.5 Hz) and 4.69 br-s, each one H related to external methylene, 3.26 (dd, Jax,ax = 11.0 , Jax,eq= 4.0 Hz, H3) geminal to oxygenated carbon, 2.03 (s, acetate methyl), 1.01 (d, J = 3 Hz, Me27), 0.99 (d, J = 3 Hz, Me26), 0.94 (s, 6H:Me18, Me30 ), 0.88 (s, Me28), 0.86 (d, J = 6 Hz, Me21), 0.79 (s, Me29) together with 0.30 (d, 4.25 Hz) and 0.53 (J = 4.25 Hz) of cyclopropane ring were observed in 1H-NMR spectrum.
Proliferation assay
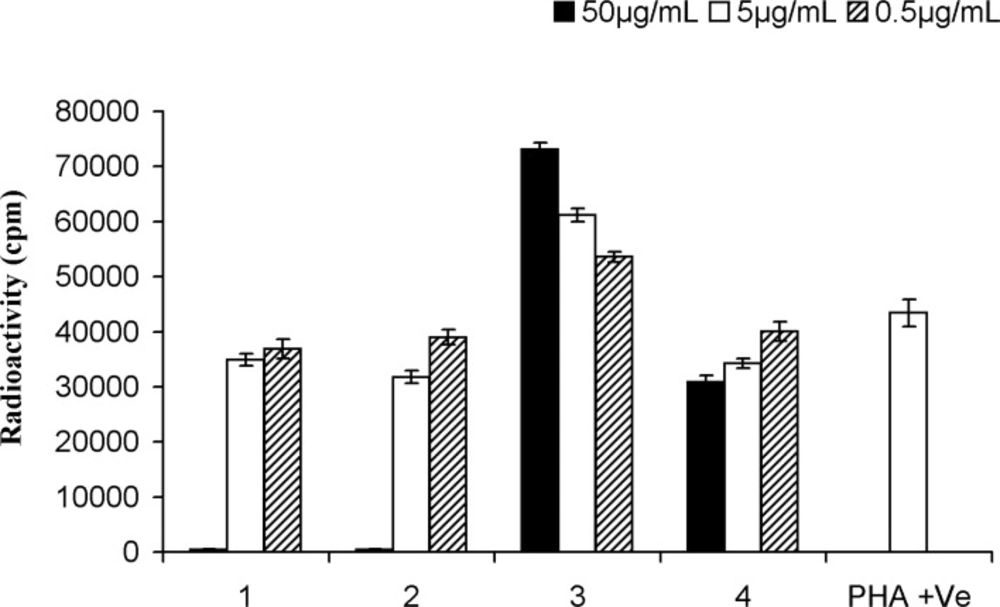
The anti-proliferation effect of the test compounds was determined by measuring the PHA-induced T-cell proliferation by determining radioactive thymidine incorporation. Comparison of pasitive, negative controls were included to assiss the activity of test compounds. cycloart-25-ene-3β, 24-diol (1) showed dose-dependent decrease in lymphocyte proliferation with IC50: 12.1 ± 0.6 μg/mL. This result was in conformity with another study by Smith-Kielland (14) which showed cytotoxic activity against Ehrlich ascites tumor cells in mice. Likewise, 24-methylene-cycloartan-3β-ol (2), presented dose dependent inhibitory effect with IC50: 10.4 ± 0.1 μg/mL agreed with other published data supporting pain-relieving activity, and anti-inflammatory effect by TPA-induced ear oedema in mice (15). Masking free OH groups of 1 and 2 by acetylation, anti-proliferative effect decreased significantly (IC50 > 50 μg/mL). On the other hand, in the case of 3 with two acetyloxy groups (3-OAc and 24-OAc), proliferation of PBLs increased by 23-25% at the low concentration (0.5 μg/mL) in comparison with PHA (5 μg/mL) as positive control. However of the higher concentration 50 and 5 μg mL− 1 of 66-70% and 36-39% increas in prolification were absorb. These results suggested that the proliferation stimulatory activity on PBLs is related to the presence of 24-OAc function while anti-proliferation effect induced by free 3-OH group (Figure 3).
Proliferation assay on peripheral blood lymphocytes of cycloartanes in Euphorbia aellenii. cycloart-25-en-3β,24-diol (1), 24-methylene-cycloartan-3β-ol (2), 3β, 24-O-diacetyl-cycloart-25-en (3), 3β-O-acetyl-24-methylene-cycloartan (4), T-cells were stimulated by phytohemagglutinin (PHA) in the presence of three different concentrations of compounds. Significance differences between the means of compounds as compared to the control (PHA +ve) were calculated by using one-way ANOVA at p = < 0.05
Docking results
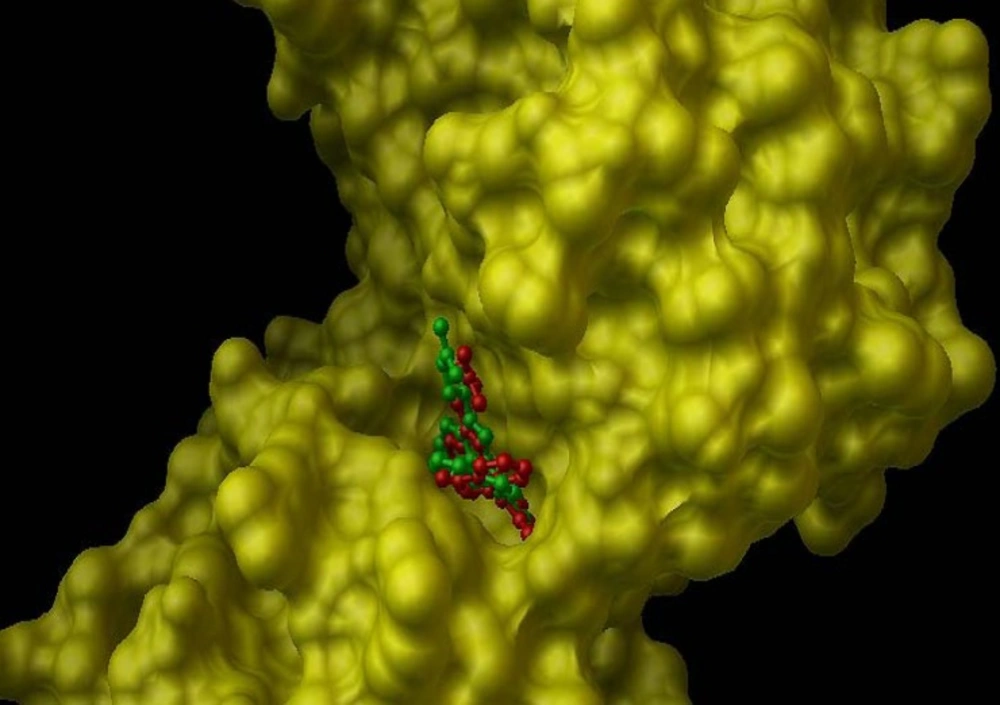
In the internal validation phase of docking, bis(indolyl) maleimide was docked onto the PKC and the lowest energy pose for docking is shown in Figure 4. Superimposing the experimental and predicted conformations, the RMSD was achieved as 1.02 A°, considered as a successful docking (16) of such ligands with PKC. Thereafter, the proposed mechanism of the action was validated by docking the compounds (1-4) in the binding site (Figure 5). All of four compounds tended to accept similar orientations, and docked into the active site of PKC. In compounds (1-2) forming hydrogen-bond interactions between 3-OH and Pro-532, could explain the antiproliferative effect of T-cell derived PKC in-vitro. Therefore, based on this structure-function study, the presence of 3-OH could be correlated with the ability to deactivate PKC and inhibited T-cell proliferation. Likewise, hydrogen bond interaction between 24-O-acetyl group (3) and Asp-330 of PKC active site could be responsible for induction of lymphocyte proliferation derived PKC in-vitro.
Conclusion
For the first time Cycloart-25-en-3β,24-diol (in high quantity) and 24-methylene-cycloartan-3β-ol could be isolated from Euphorbia aellenii. Their immunomodulatory effects suggested that the proliferation stimulatory activity on PBLs is related to the presence of 24-OAc function while anti-proliferation effect induced by free 3-OH group. The SAR studies on PKC confirmed these results and provided further support for the role of PKC in transduction of activation signals in T-cells.