Introduction
Current available anticonvulsant drugs are able to efficiently control epileptic seizures in about 50% of the patients; another 25% may show improvement whereas the remainder does not benefit significantly (1). Furthermore, undesirable side effects of the drugs used clinically often render treatment difficult so that a demand for new types of anticonvulsants exists. One of the approaches to search for new antiepileptic drugs is the investigation of naturally-occurring compounds, which may belong to new structural classes.
Glycyrrhiza glabra L., Fabaceae, is a tall, erect perennial herb with branched stalks which grow to 1.5 m. G. glabra is a native of South-East Europe and South-West Asia, which includes Iran. It is among the world’s most ancient herbal remedies with a wide range of pharmacological activities including expectorant, antitussive, emollient, anti-inflammatory, antipyretic, antiviral, antibacterial, antiprotozoal, hepatoprotective, antitumor, vasorelaxant, antiplatelet aggregation, immunomodulatory, endocrinological, antidepressant, memory enhancing, sedative, muscle relaxant (2) and antifungal effects (3). The root of G. glabra has also been studied for anticonvulsant effect and was found to be effective (4, 5). There are several varieties of G. glabra of which, two varieties including var. violacea and var. glandulifera grow in iran (2). There is no report regarding the possible anticonvulsant effects of the different varieties of G. glabra. On the other hand, some compounds believed to be responsible for anticonvulsant effects of the root of G. glabra, such as flavonoids which are also found in G. Glabra leaves (6-8). If the leaves are also proven to be effective, considering the perennial nature of this plant together with the easy harvest of the leaves, which provide a high yield of readily accessible target tissue, they could be a renewable source of bioactive anticonvulsant material. Therefore, in this study the possible anticonvulsant and toxic effects of the leaves of G. glabra var. glandulifera were assessed in mice.
Experimental
Plant materials
Leaves of G. glabra var. glandulifera were collected from Borujen (Chahar Mahall and Bakhtiari province, Iran) in May 2007. G. glabra var. glandulifera was authenticated by Soroush Sardari and a voucher specimen (No. 74-86) was deposited in the herbarium of Pasteur Institute of Iran.
Chemicals
Pentylenetetrazole (PTZ), phenytoin sodium and ethosuximide were purchased from Sigma-aldrich (Pool, UK). N-hexane, Tween 80, dimethyl sulfoxide (DMSO), methanol, ethanol, dichloromethane, ethyl acetate, toluene, antimony trichloride, dragendorrf’s reagent, potassium hydroxide, glacial acetic acid, vanillin, sulphuric acid, ferric chloride, hydrochloric acid and sodium hydroxide were all from Merck (Darmstadt, Germany). PTZ, phenytoin sodium and ethosuximide were dissolved in saline solution (0.9%). The extract and the fractions were dissolved in Tween 80 (25%) : DMSO (2 : 1 v/v) mixture and used freshly.
Extract preparation
The air-dried leaves (100 g) of the plant were ground and extracted at the room temperature for 48 h by percolation method using 80% ethanol (900 mL). The extract was then concentrated with a rotary evaporator apparatus at temperature not exceeding 40°C. The yield of the extract was 45% (w/w). The extract was stored at 4°C throughout experiments.
Fractionation
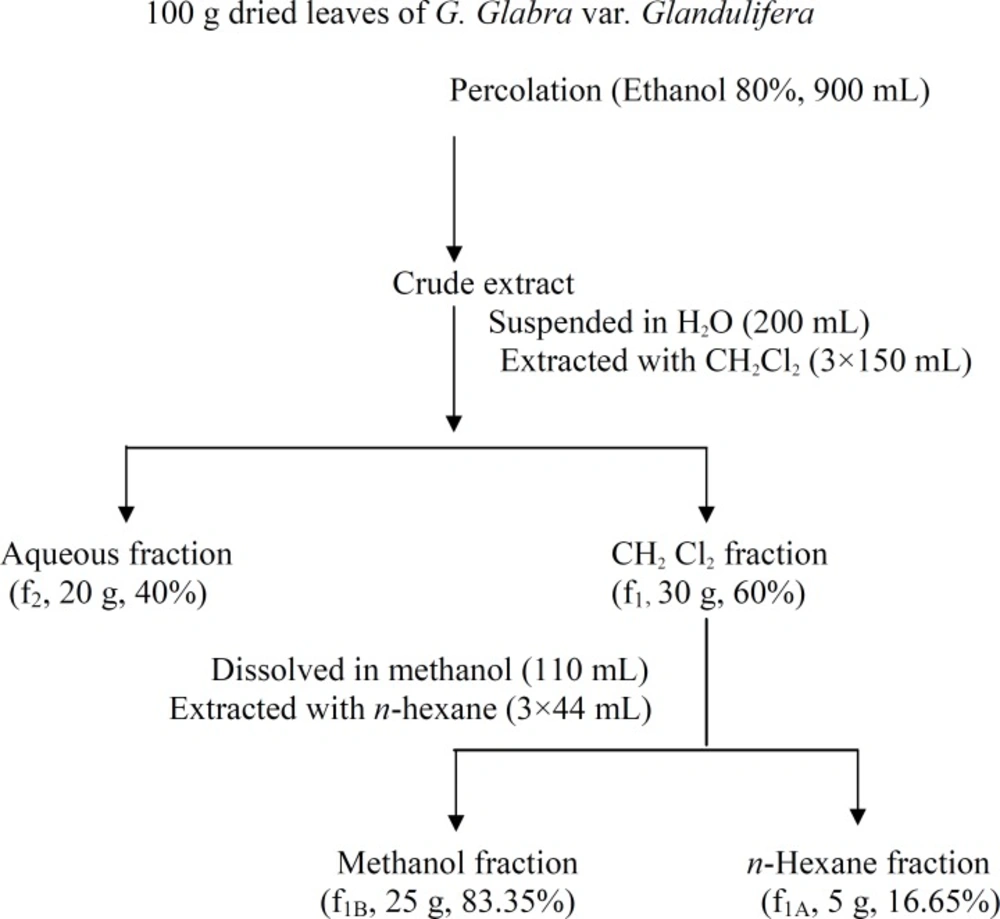
The crude extract was suspended in 200 mL distilled water and extracted with dichloromethane for three times (each time 150 mL). The dichloromethane (f1) and the aqueous (f2) parts were collected separately and dried by rotary evaporator at 40°C. f1 was further partitioned by methanol (110 mL, f1A) and n-hexane (44 mL, f1B) (Figure 1). Both parts were dried by rotary evaporator at 40°C.
Preliminary phytochemical screening
The crude extract and the fractions were screened for the presence of triterpenes/sterols, alkaloids, flavonoids, anthraquinones, anthrones, coumarines, valepotriates, essential oil and tannins by thin layer chromatography using silica gel G (Merck, Germany) plates of 0.25 mm thickness (9). The extract and fractions were dissolved in Tween 80 (25%) : DMSO (2 : 1v/v). Development was carried out with ethyl acetate : methanol : water (100 : 13.5 : 10 v/v/v) and ethyl acetate : toluene (93 : 7). After development, the plates were sprayed with the following reagents for detecting the respective classes of compounds: antimony trichloride (triterpenes/sterols), dragendorrf’s reagent (alkaloids), potassium hydroxide (anthraquinones, anthrones and coumarins), hydrochloric acid-glacial acetic acid (valpotriates), vanillin-sulphuric acid (essential oil) and ferric chloride (tannins). Reagents were prepared according to Stahl (10). Detection was carried out visually in visible light and under the UV light (λ = 365 nm).
Animals
Male NMRI mice (20-28 g, Pasteur Institute of Iran) were used. The animals were housed in standard cages with free access to food (standard laboratory rodent’s chow) and water. The animals’ house temperature was maintained at 23 ± 1°C with a 1 h light/12 h dark cycle (light on from 06:00 to 18:00). The study was approved by the ethics committee of Pasteur Institute of Iran and conforms to the European Communities Council Directive of 24 November 1986 (86/609/EEC). All animals’ experiments were carried out in such a way that minimized the number of animals and their suffering. Each animal was tested once. All the injections were intraperitoneal (IP) in volume of 0.1 mL/10 g of mice body weight.
PTZ-induced seizures
The minimal IP dose of PTZ at which 99% of the animals showed general clonus was determined by a dose-percent effect curve. General clonus was considered as the criteria of clonic seizure which characterized by clonus of four limbs with transient loss of righting reflex (11). The dose (60 mg/Kg) was then injected to 18 groups of 10 mice each were pretreated IP 30 min before that, with the crude extract (1, 1.5, 2, 3 and 4 g/Kg), f1 (1, 1.5 and 2 g/Kg), f2 (2 and 4 g/Kg), f1A (1, 2 and 3 g/Kg), f1B (1 and 1.5 g/Kg), ethosuximide (150 mg/Kg, as positive control), saline (10 mL/Kg, as control) and the solvent of the extract and the fractions (10 mL/Kg, as control). If no general clonus occurred during a 30 min period of observation, the animals were considered protected.
MES-induced seizure
Electro-convulsive shock, inducing Hind Limb Tonic Extension (HLTE) in 99% of the animals (11) was previously determined (12). The electrical stimulus (50 mA; 50 Hz; 1 sec duration) was applied through ear-clip electrodes using a stimulator apparatus (MGH-777, Development of Electronic Industry, Iran). Five groups of 10 mice each were pretreated IP with the crude extract (3 and 4 g/Kg), phenytoin (25 mg/Kg, as positive control), saline (10 mL/Kg, as control) and the solvent of the extract and fractions (10 mL/Kg, as control). After 30 min the animals received transauricular electroshock. Abolition of HLTE within 10 sec after delivery of the electroshock was the criterion for anticonvulsant effect.
Acute toxicity
Seven groups of 10 mice each were treated IP with the solvent of the extract and the fractions (10 mL/Kg, as control), the crude extract (1.5, 2, 3 and 4 g/Kg), f1 (2 g/Kg) and f1A (3g/Kg). The mortality rate was recorded after 24 h.
Data analysis
The dose of the extract required to produce an anticonvulsant effect (ED50) or death (LD50) in 50% of the animals and its associated 95% confidence limit was calculated by the method of Litchfield and Wilcoxon (13) using a commercial computer program (GRAPHPAD INSTAT 3, version 2003). The therapeutic index (TI) of the extract was calculated via dividing the LD50 by the ED50. Data obtained from the convulsive tests were expressed as the percentage of the animals showing convulsions and Fisher’s exact test was used to analyze the data. P-value less than 0.05 was the critical criterion for statistical significance.
Results and Discussion
Anticonvulsant activity
The crude extract and f1 fraction showed anticonvulsant activity (Table 1). The ED50 value of 2.11 g/Kg and 1.30 g/Kg were obtained for the crude extract and f1 fraction, respectively. However, f2, f1A and f1B fractions had no protective effect against clonic seizures induced by PTZ (Table 1).
| Treatment | Dose | Incidence of clonic seizures (%) |
|---|---|---|
| Control 1 | 10 mL/Kg | 100 |
| Control 2 | 10 mL/Kg | 80 |
| Ethosuximide | 150 mg/Kg | 0٭٭٭ |
| Crude extract | 1 g/Kg | 80 |
| Crude extract | 1.5 g/Kg | 60 |
| Crude extract | 2 g/Kg | 40* |
| Crude extract | 3 g/Kg | 30٭٭ |
| Crude extract | 4 g/Kg | 20٭٭ |
| f1 fraction | 1 g/Kg | 80 |
| f1 fraction | 1.5 g/Kg | 30٭٭ |
| f1 fraction | 2 g/Kg | 20٭٭ |
| f2 fraction | 2 g/Kg | 70 |
| f2 fraction | 4 g/Kg | 100 |
| f1A fraction | 1 g/Kg | 80 |
| f1A fraction | 2 g/Kg | 80 |
| f1A fraction | 3 g/Kg | 70 |
| f1B fraction | 1 g/Kg | 80 |
| f1B fraction | 1.5 g/Kg | 80 |
The crude extract up to the dose of 4 g/Kg did not show any anticonvulsant effect against tonic seizures induced by MES (Table 2).
| Treatment | Dose | Incidence of tonic seizures (%) |
|---|---|---|
| Control 1 | 10 mL/Kg | 100 |
| Control 2 | 10 mL/Kg | 80 |
| Phenytoin | 25 mg/Kg | 0٭ |
| Crude extract | 3 g/Kg | 100 |
| Crude extract | 4 g/Kg | 100 |
Mortality
At the anticonvulsant doses, the crude extract and fractions had lethal effects on the animals (Table 3). The LD50 value of 3.03 g/Kg was obtained for the extract.
| Treatment | Dose | Incidence of mortality (%) |
|---|---|---|
| Control | 10 mL/Kg | 0 |
| Crude extract | 1.5 g/Kg | 0 |
| Crude extract | 2 g/Kg | 20 |
| Crude extract | 3 g/Kg | 30 |
| Crude extract | 4 g/Kg | 90 |
| f1 fraction | 2 g/Kg | 90 |
| f1A fraction | 3 g/Kg | 80 |
Preliminary phytochemical analysis
The constituents of the ethanol extract and the fractions are demonstrated in Table 4. The extract and f1 contain triterpenes/sterols, flavonoids, alkaloids, anthraquinones and tannin. Anthrones, coumarines, valepotriates and essential oil were not found in any of the extract or fractions.
| Extract or fraction | Ethanol extract | f1 | f2 | f1A | f1B |
|---|---|---|---|---|---|
| Compound | |||||
| Triterpenes/sterols | ++ | ++ | - | - | + |
| Alkaloids | + | + | - | + | - |
| Flavonoids | + | + | ++ | + | + |
| Anthraquinones | ++ | + | - | - | + |
| Anthrones | - | - | - | - | - |
| Coumarines | - | - | - | - | - |
| Valepotriates | - | - | - | - | - |
| Essential oil | - | - | - | - | - |
| Tannin | + | ++ | +++ | - | + |
The results of the present study indicate that the crude extract of G. glabra var. glandulifera blocks clonic seizures induced by PTZ and the ED50 value of 2.11 g/Kg was obtained for the extract. In order to pick out the anticonvulsant components of the leaves, the ethanol extract was fractionated. Bioactivity-guided fractionation showed that the active anticonvulsant principle (s) were non-polar as the activity was observed in dichloromethane fraction (f1) and not in the aqueous fraction (f2). However, at the anticonvulsant doses, f1 was lethal for the animals. Therefore, it seems that in f1 fraction, both toxic and anticonvulsant principles are present. By further fractionation neither n-hexane (f1A) nor methanol (f1B) sub-fractions had anticonvulsant activity. Thus, anticonvulsant mixtures or combination of principles that are responsible for the activity are absent in sub-fractions (f1A and f1B). The phytochemical tests performed in this study revealed the presence of triterpenes/sterols, alkaloids, flavonoids, anthraquinones and tannins in the leaf extract and f1 fraction. However, in f2, triterpenes/sterols, alkaloids and anthraquinones, in f1A, triterpenes/sterols, anthraquinones and tannins, and in f1B, alkaloids, were not found. Triterpenoids, flavonoids and alkaloids are detected in the root of G. glabra (2, 14) and their anticonvulsant activity has been demonstrated previously (15-18). Therefore, the anticonvulsant activity of the leaf extract and f1 could be attributed to the combined activity of triterpenoids, flavonoids and alkaloids present in the plant. Although f1A fraction is mainly composed of lipophilic substances, the phytochemical investigation showed only traces of certain secondary metabolites such as flavonoids, which are most likely inactive for anticonvulsant property. The rest of this fraction could be made from primary metabolites of lipid nature that are not expected to show such bioactivity. On the other hand, the highest concentrations of triterpenes together with four other categories of phytochemicals present in the crude extract and f1, corresponded to anticonvulsant activity. Thus, it seems that the absence of such pattern of phytochemicals in n-hexane fingerprint of chemicals could be the reason of non-effectiveness of f1A fraction against seizures.
We obtained the LD50 value of 3.03 g/Kg for the extract, which is close to the ED50 value and the calculated TI value of 1.43 g/Kg for the leaf extract was narrow. It seems that the toxicity of the extract and the fractions are the result of the combined toxicity of the constituents such as alkaloids, anthraquinones and tannins whose toxicity has been reported by many researchers (19-31).
The anticonvulsant effect of an ethanol extract obtained from the roots of G. glabra has been previously reported (4). In that study, ED50 value of about 20 mg/Kg obtained for the extract against clonic seizures induced by PTZ in mice. The root ethanol extract of G. glabra has wide TI and low toxicity where even at the doses 50 times higher than ED50, no death or toxicity has been observed in mice (4).
In conclusion, the leaf ethanol extract of G. glabra var. glandulifera possesses protective effect against PTZ-induced clonic seizures. The extract however, has narrow TI. Therefore, compared to the roots, the leaf has weak potential to be considered as a source for anticonvulsant compounds. Investigation on the possible anticonvulsant activity of the leaves of the other varieties of G. glabra grown in Iran (e.g., var. violacea) is suggested.