Introduction
Antituberculosis drug-induced hepatitis is one of the most prevalent drug-induced liver injuries (1). Rifampin (RIF), isoniazid (INH), pyrazinamide (PZA) and ethambutol (EMB) are first line chemotherapeutic agents in treatment of tuberculosis (TB) (2-5). Among these drugs, INH is one of the major drugs incriminated in this hepatotoxicity (6). Hepatotoxicity of INH can be presented as a mild transient elevation in aminotransferases in 10 to 20% of tuberculosis patients to rare cases of overt hepatitis (7)
Metabolism pathways and toxic metabolites of anti-TB agents play a central role in hepatotoxicity of these agents (8). INH is mainly inactivated by n- acetyltransferase-2 (NAT2)-mediated acetylation, resulting in acetyl-INH, which is hydrolyzed to acetylhydrazine and isonicotinic acid (9).
Another important factor in INH-induced hepatotoxicity is genetic variations (6, 9). There are large variations in the INH acetylation capacity suggesting a pharmacogenetic polymorphism of NAT2 (10, 11). This variability in the acetylation capacity results from a wide range of n- acetylation activity and has been characterized by bimodal or trimodal distribution (12,13). The frequency of slow and rapid acetylators differs among ethnic populations (14-17).
Although primary studies had suggested that the fast acetylators are more susceptible to developing INH induced hepatotoxicity (17, 18), recent studies showed that the slow acetylators are more vulnerable to INH induced hepatotoxicity (19, 20). Still the association between acetylation phenotype and drug-induced hepatotoxicity is controversial (6, 10).
The goal of this study was to evaluate relationship of acetylator phenotype and the incidence of antituberculosis drugs-induced hepatotoxicity in Iranian pulmonary TB patients.
Experimental
This study was a prospective, cross-sectional one. A total of 50 unrelated patients (age > 18) with newly diagnosed pulmonary TB from the infectious diseases ward from Imam Referral Hospital affiliated to Tehran University of Medical Sciences (Tehran, Iran) were entered into the study from September 2006 to September 2008.
Standard tuberculosis treatment regimen in our country is based on WHO recommendation and includes: INH (5 mg/Kg), RIF (10 mg/Kg), PZA (25 mg/Kg), EMB (15 mg/Kg) for the first 2 months followed by INH and RIF daily for 4 additional months. WHO TB diagnosis criteria including (I) a positive culture for mycobacterium TB or (II) negative culture patient with clinical and radiological features consistent with TB and response to anti-TB treatment or (III) histological findings consistent with TB and response to anti-TB treatment were used for diagnosis of TB in our hospital (4). Patients with human immunodeficiency virus (HIV) infection, hepatic insufficiency (ALT or AST > 2 × upper limit normal or clinically symptoms of liver diseases such as jaundice and ascites) and renal insufficiency (creatinine clearance less than 50 mL/min based on Cockcroft-Gault equation), history of smoking (only positive based on individual expression) and chronic alcohol consumption (persons that have history of regular alcohol drinking at least 30 g alcohol daily for 6 months before hospital admission) excluded from the study.
For all the patients involved in the study, a complete history and physical examination were administered and patients’ demographic characteristics, history of smoking, alcohol drinking, drug abuse, concomitant diseases and drugs, status of viral infections and other treatment information were collected.
The liver function tests (LFT) including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphates (ALP), direct and total bilirubin and clinical symptoms of drug-induced hepatotoxicity such as anorexia, abdominal pain, nausea and vomiting and right upper quadrant pain were monitored prior to and during anti-TB therapy.
The causality of a drug-induced hepatotoxicity was determined using the Roussel Uclaf Causality Assessment Method (RUCAM) (21).
Sampling
At the beginning of the anti-TB treatment course and after the overnight fasting, each patient received 300 mg INH (one tablet) orally and then 3 h later, 5 mL of venous blood sample was collected into an EDTA tube and immediately centrifuged. The plasma samples were then separated and kept frozen at -70°C, waiting for analysis. First doses of other anti-TB drugs were started after blood sampling.
Plasma INH and Acetyl-INH were measured by a gradient HPLC method (22). Acetylator phenotype was determined from the metabolic ratio (MR) of Acetyl-INH to INH in the plasma samples (23). MR was used to classify the subjects as slow (MR < 1.0) or fast acetylators (MR > 1.0) (23).
Hepatotoxicity was defined as (I) the increased levels of liver transaminases more than three times above the normal value (< 40 UL−1 for AST and ALT) with any other clinical signs and symptoms, (II) and the elevation of transaminases more than five times above the normal limit if patients had no symptoms (24). For assessing the effect of age on anti-TB induced hepatotoxicity, patients were divided into two groups: those whose age were < 35 (group A) and those whose age were ≥ 35 (group B) (25).
Data analysis
Data obtained from our study was expressed as mean-values ± SD, numbers or percentages and was analyzed using the Statistical Package for Social Sciences version 16.0. Group comparisons for categorical variables were carried out using the chi-square (x2) test and Fisher’s exact test. All statistical tests were based on a two-tailed probability, and a p-value ≤ 0.05 was considered significant.
Results and Discussion
The age of the patients ranged from 18 to 86 years with a mean of 47.8 ± 21.9 years. There were 28 (56%) males and 22 (44%) females. Fourteen patients (28%), 8 of 28 males (28.6%) and 6 of 22 females (27.3%) developed hepatocellular type liver injury. The mean age of the patients who developed hepatotoxicity, was 43.1 ± 22.7 years (the age range = 19-66 years). Among the 50 patients, 20 (40%) were slow acetylators and 30 (60%) were fast acetylators.
For evaluation of causality, we used RUCAM (21). Hepatotoxicity induced by anti-TB drugs was classified as probable based on the causality assessment method. Hepatotoxicity was manifested in 9 of 20 slow acetylators (45%) and only in 5 of 30 rapid acetylators (16.7%). The mean duration of treatment before the start of hepatotoxicity was 14.6 ± 6.4 days. The application of x2 test showed a significant difference between the acetylation phenotype and hepatotoxicity (x2 = 4.778, and p = 0.03). Data are shown in Table 1.
| Hepatotoxicity | Total | ||||
|---|---|---|---|---|---|
| No | No | Yes | |||
| Phenotype | Slow | Count | 11 | 9 | 20 |
| % within phenotype | 55.0% | 45.0% | 100.0% | ||
| Fast | Count | 25 | 5 | 30 | |
| % within phenotype | 83.3% | 16.7% | 100.0% | ||
| Total | Count | 36 | 14 | 50 | |
| % within phenotype | 72.0% | 28.0% | 100.0% | ||
Frequencies of n- acetyl transferase 2 phenotype and anti-tuberculosis drug-induced hepatotoxicity in Iranian pulmonary tuberculosis patients
Patients were divided into two groups, due to their ages. From 22 patients in group A (age < 35), seven developed hepatotoxicity, 4 of them were slow acetylators and 3 patients were fast acetylators. Twenty eight patients were ≥ 35 years old and consequently were categorized in group B. Seven patients (5 slow and 2 fast acetylators) in this group developed hepatotoxicity. Our data is summarized in Table 2. There was not statistically significant difference in the frequency of INH-induced hepatotoxicity between group A and B (x2 = 0.284, and p = 0.59). Furthermore, there was no association between sex and hepatotoxicity in our study using Fisher’s exact test (p = 1.00).
| Total | Hepatotoxicity | ||||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| Acetylation phenotype | Total | Group | A | 22 | 15 | 7 | |
| B | 28 | 21 | 7 | ||||
| slow | Group | A | 9 | 5 | 4 | ||
| B | 11 | 6 | 5 | ||||
| fast | Group | A | 13 | 10 | 3 | ||
| B | 17 | 15 | 2 | ||||
Summary of anti-tuberculosis drug-induced hepatotoxicity based on the age group and n- acetyl transferase-2 acetylation phenotype
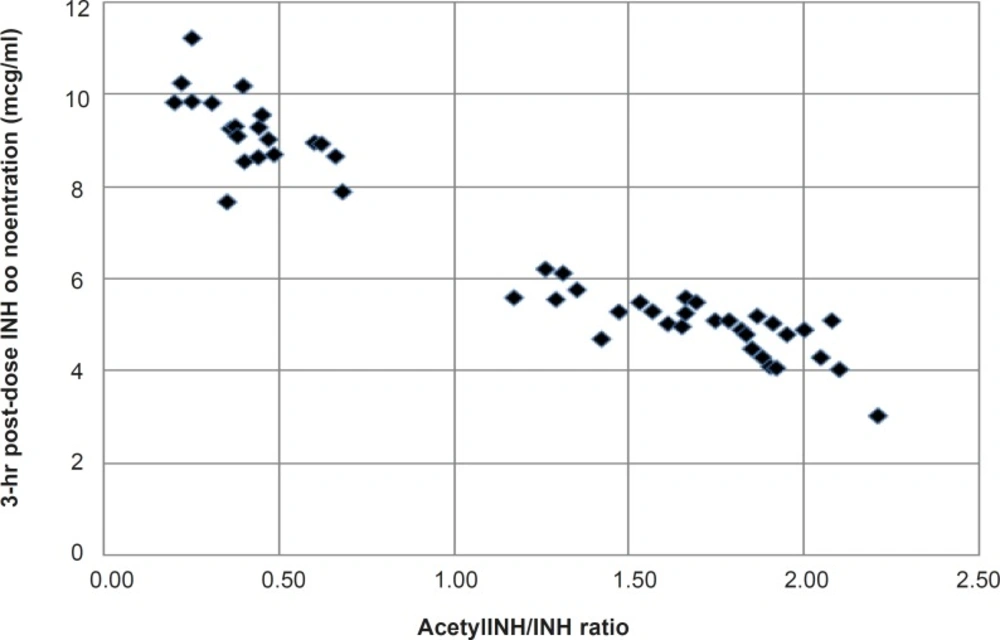
Frequency histogram of metabolic ratio (acetyl INH/INH) showed an apparent bimodal distribution with an apparent antimode of acetylation ratio of 1.0, separating the slow acetylators from the fast ones (Figure 1).
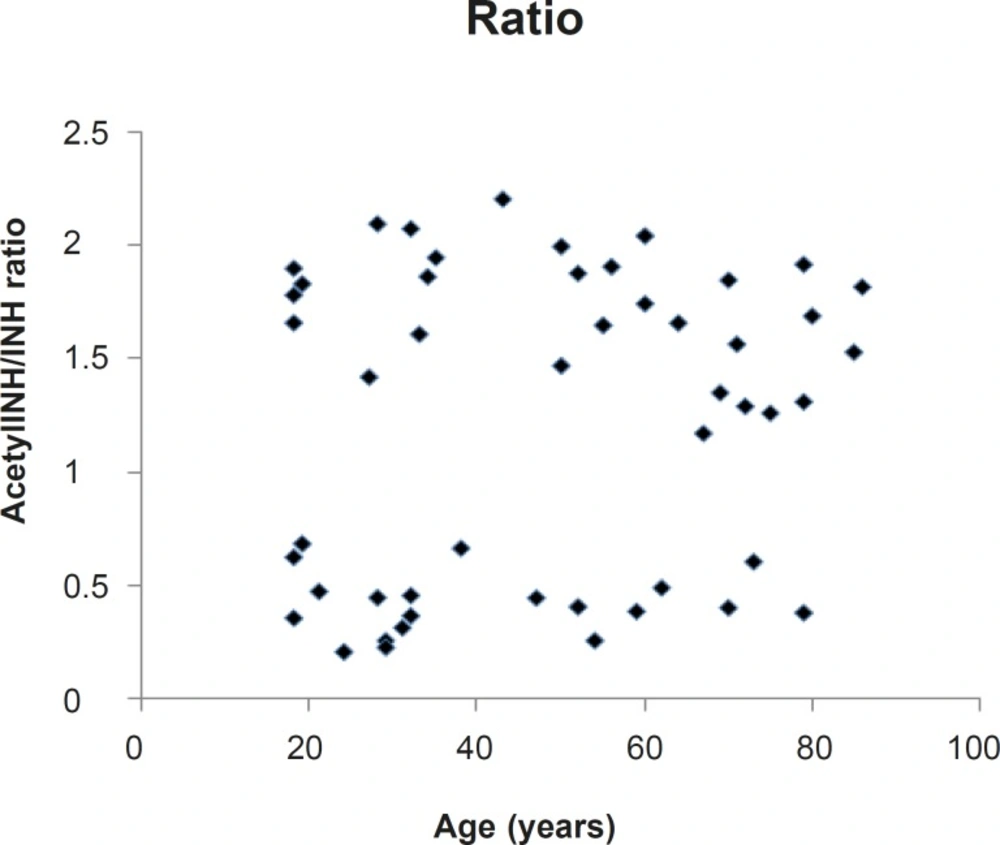
Moreover, as shown in Figure 2, there is a lack of correlation between the metabolic ratio (acetyl-INH/INH) and the age of the subjects (r = 0.16).
Various proportions of rapid and slow acetylators of different populations in relation to the ethnicity and geographic location were reported (26). There are limited reports about the pattern of acetylation in Iranian population. We have determined the frequency of NAT2 polymorphisms, NAT2 acetylation profile and also its relationship with the occurrence of anti-TB drug induced hepatotoxicity. Our major finding is the association of the slow acetylation profile with anti-TB drug-induced hepatotoxicity in a population of TB patients from Iran, which is in accordance with results described by other researchers (6, 27). The present findings contrast sharply with the observations of Mitchell, J.R., et al. (28), who reported increased susceptibility of anti-TB drug hepatotoxicity in fast acetylators. In contrast, a number of other studies did not find any relationship between acetylator status and drug-induced hepatotoxicity (9, 29). This clear discrepancy among the results of previous studies on acetylation status and anti-TB hepatotoxicity may be due to the different designs of the studies, especially in terms of the methodology for NAT2 typing, the anti-TB drugs used, and the criteria for defining anti-TB drug hepatotoxicity.
The incidence of antituberculosis drug-induced hepatic dysfunction, ranged from 1% to 36% (6, 9, 28). The rate of anti-TB induced hepatitis in this study was 28%, comparable with 27.7% that reported in Iranian patients by Sharifzadeh M., et al. (24). The wide variation of incidence may depend on the definition of hepatic dysfunction, study design, race, sex, and different concomitant use of antituberculosis drugs. There are possible causes for the higher incidence of antituberculosis drug-induced hepatitis in our study in comparison with some other studies (1). Unlike our prospective regular monitoring of liver enzyme test results, Kopanoff DE et al. (30) checked the liver function test results only on clinical indication, not for routine monitoring. This may underestimate the incidence of hepatic dysfunction (4). Some studies have evaluated and reported only INH hepatotoxicity (9, 31) but it was documented that concomitant use of other hepatotoxic drugs such as RIF and PZA can increase the incidence and severity of INH-related hepatic dysfunction (32). Asian population may have increased susceptibility to anti-TB related hepatotoxicity. This is supported by the following observations. The highest incidence (36%) of anti-TB induced hepatitis was reported from Japan (27). The reported incidence of hepatotoxicity in Iranian patients was 27.7% (24).
The prevalence of NAT 2 phenotype in a sample of healthy Iranian individuals including 88 samples was 32.9% slow, 48.9% intermediate, and 18.2% rapid using PCR-RFLP method (33). In another study using PCR-RFLP method, the frequency of slow, intermediate and rapid acetylator phenotype was 49.4%, 41.5%, and 9.1% respectively in 229 unrelated healthy subjects from the general Tehran population (34). Frequency of slow acetylator phenotype in these studies is approximately near to our finding (32.9%, and 49.4% vs. 40%). These studies have classified subjects genotypically as slow, intermediate, and fast. It seems that genotypically classified intermediate subjects phenotypically classified as fast in our study. The discrepancy probably originated from the difference in the methodology used, variations in study subjects (healthy individuals versus patients), the effects of unidentified genetic or environmental factors, and incomplete correlation between reported acetylator phenotypes and corresponding NAT2 genotypes.
In another study, polymorphism of NAT 2 was studied in 74 unrelated healthy Iranian volunteers, using sulfamethazine as metabolic probe. The frequency of slow acetylators, determined by using free and total plasma sulfamethazine concentrations, was 78.4% (35). We used INH as metabolic probe. The major finding is that phenotype assignment was dependent on the probe substrate (36). In addition to INH and sulfamethazine, other acetyl-transferase substrates such as caffeine (37) and dapsone (38) have been used as phenotypication probe.
On the other hand, in previous studies over Iranian population, subjects were healthy volunteers, whereas the TB ones were studied in here.
Age was not significantly different among the patients with and without hepatotoxicity. Our findings are in agreement with the previous study (9), but another study reported old age as a risk factor for development of hepatotoxicity during the treatment of TB (39).
For women, it was reported increased risk of hepatotoxicity but this did not achieve statistical significance (40). Our findings also show no increased risk in women.