Introduction
During physiological and pathological hemolysis, hemoglobin (Hb) is released in plasma from red blood cells or their precursors (1). In healthy individuals physiological hemolysis accounts for ~10% of red blood cell destruction in the circulation, known as intravascular hemolysis (2). Moreover, the free-Hb may also increase by pathological hemolysis during some diseases such as hepatitis (3). The free-Hb represents a highly toxic substance, unless rapidly cleared from the circulation. This toxicity arises from the heme-iron, which can react with endogenous hydrogen peroxide to produce free radicals that may cause severe oxidative tissue damage and consequent disruption of cell function (4-6). On the other hand, iron content may decrease in the body because of glumerular filtration of the relatively small free-Hb molecules (7).
Haptoglobin (Hp) is a plasma glycoprotein produced and released mostly by hepatocytes into the circulation. Haptoglobin irreversibly forms a soluble complex with free-Hb via non-covalent bonds. This binding is one of the strongest non-covalent interactions reported in biology (8-10). The formation of Hp-Hb complex has two main functions during hemolysis. As evidenced by studies in Hp knockout mice, it protects tissuses against heme-mediated oxidative damages, in particular in the kidneys (11, 12). The other is reduction of glomerular filtration of the free-Hb molecules after Hp-Hb complex formation (7). Eventually the Hp-Hb complex can be internalized by macrophages that express receptor for the complex (13) in order to recycling iron.
In various diseases such as infectious diseases, oxidative stress occurs secondary to the initial disease but plays an important role in immune or vascular complications (14). Interestingly, the relative antioxidant effect of some β-lactam antibiotics such as ampicillin on oxygen-reactive species (ROS) has been reported and a possible therapeutic role for β-lactam agents in protecting host tissues from oxidative damage has been proposed (15). With regard to the extensive and strong antioxidant activity of Hp-Hb complex mentioned earlier, this complex is considered as an active part of body antioxidant defense system (16-18). Moreover, since the complex level in serum rises considerably after various diseases such as bacterial infection (19-21), it has been used as a biomarker in clinical diagnosis of those diseases (22, 23). Hp level in serum has also been subject of many researches in the presence and absence of antibiotics (24-27) which used as treatment of bacterial infections. But there has been a lack of information about antioxidant activity of Hp-Hb complex in the presence of antibiotics, especially β-lactam antibiotics which are related to host oxidative stress.
As it has been mentioned before, heme-iron of free-Hb can react with endogenous hydrogen peroxide and produce free radicals (4), while Hp-Hb complexes have an ability to remove these hydrogen peroxide molecules by peroxidase activity (28). In this study, for the first time, kinetic analysis of peroxidase activity of Hp (2-2)-Hb complex has been performed in oxidative status induced by different concentrations of hydrogen peroxide. Moreover, effect of two β-lactam antibiotics (ampicillin and coamoxiclav) on the kinetics has been investigated as third parameter which is effective on oxidative condition (15). The data indicated that both antibiotics increased peroxidase activity of Hp (2-2)-Hb complex.
Experimental
Materials
Hydrogen peroxide
A solution of hydrogen peroxide, 0.5M was prepared in phosphate buffer 50 mM and pH 7.5. Peroxide solution is discarded after 30 min and if necessary a fresh dilution is prepared (29).
Guaiacol reagent
A buffered solution of guaiacol, 0.03 M, is prepared as follows: 1.86 g of guaiacol and 50 mL of acetic acid are added to 400 mL of water. The volume is made up to 500 mL with water. The guaiacol reagent is stable for several weeks when stored in the cold (29).
Met-hemoglobin solution
Hemoglobin solution was prepared at a concentration of 10 mg/mL in phosphate buffer 50 mM and pH 7.5. An equal volume of 0.4 mM potassium ferricyanide is added to convert completely the oxy-hemoglobin to met-hemoglobin. The met-hemoglobin solution is then carefully diluted using the same buffer to 0.03 mM (29).
Haptoglobin 2-2 solution
A solution of Hp 2-2 (0.03 mM) was prepared in phosphate buffer 50 mM, pH 7.5 and the concentration of the solution was determined using reported extinction coefficient of haptoglobin 2-2 (ε mM-1 58.65). The molar amount of Hp2-2 was based on its monomer properties because each Hp2-2 monomer is thought to be capable of binding a single hemoglobin molecule (30, 31).
Preparation of Hp (2-2)-Hb complex
Hp (2-2)-Hb complexes were formed by mixing 50 mL of each above mentioned solution of Hp2-2 and Hb, incubating for 30 min at room temperature with gentle agitation to ensure that all Hp molecules was saturated with Hb.
Enzymatic activity assay
Peroxidase activity of Hp (2-2)-Hb complex was assayed by following increase of absorption of produced tetraguaiacol as the second substrate of Hb-Hp complex at 470 nm and 37˚C by UV-Vis spectrophotometer (CICEL Model 9000) (32). The assay mixture contained Hp (2-2)-Hb complex 64 nM, 0.03 M guaiacol and different concentration of H2O2 in phosphate buffer 50 mM, pH 7.5 in 1 mL final volume. All components except for Hp-Hb complex were added directly to a quartz cuvette and mixed by inverting 6 times. Five micro liters Hp-Hb solution was then added to the mixture, and the cuvette was inverted three times to mix the ingredients. The zero time point for following A470 was designated as the time at which Hb-Hp complex was added to the solution. For each concentration of hydrogen peroxide, the reaction was performed at least 3 separate times. To investigate the effect of antibiotic assay mixtures contained 14 µg/mL ampicillin and coamoxiclav (20 g/mL amoxicillin, and 4 g/mL clavolonic acid) which corresponded to their maximum concentrations in the plasma after injection.
Determination of enzymatic parameters
Maximum initial velocities (Vmax) were obtained graphically using appropriate saturation curves. The amount of S50 (a concentration of H2O2 in which velocity of enzymatic activity is half of Vmax) were obtained from the H2O2 concentration related to cross point of Hill plot to X-axis. The amount of (CLmax) (maximum clearance) and Smax (a concentration of H2O2 in which CLmax occurs) were graphically obtained using clearance plots.
Calculation of V-cal
Calculated initial velocity (V-cal) was obtained using Hill equation:
Where Vmax is maximum initial velocity, S50 is the substrate concentration corresponding to half of Vmax, m is Hill coefficient (slope of Hill plot) and S is various substrate concentrations (33).
Results and discussion
Although several physiological roles have been referred to Hp, the primary function of Hp still appears to be binding to free-Hb and its antioxidant activity, therefore protecting from heme-catalyzed oxidative stress and facilitating Hb-uptake by macrophages. Haptoglobin as an acute phase plasma glycoprotein (22) has two allelic sites producing three phenotypes: Hp1-1, Hp2-2 and Hp1-2. Individuals with phenotype1-1 and 2-2 have maximum and minimum antioxidant activity, respectively. Consequently, patients with Hp2-2 phenotype who suffer from chronic diseases which are related to oxidative stress are more susceptible to cardiovascular disorders (34). According to some studies less antioxidant activity of Hp2-2 can be compensated with antioxidant effects of some additives such as glutathione peroxidase (35) and vitamin E (36).
Antioxidant activity of Hp-Hb complex is an important factor, not only in chronic diseases, but also in some acute infections with considerable increase in serum level of Hp (19-23). With regard to the wide-spread use of antibiotics for treatment of various infectious diseases, their effects as an additional factor on antioxidant property of Hp-Hb complex can be of interest (24-27), especially if it is considered that, importantly, antibiotic susceptibility of bacteria is also related to oxidative status (37-39). Here we have studied on the effect of the presence of two β-lactam antibiotics to investigate probable increasing in peroxidase activity of Hp (2-2)-Hb.
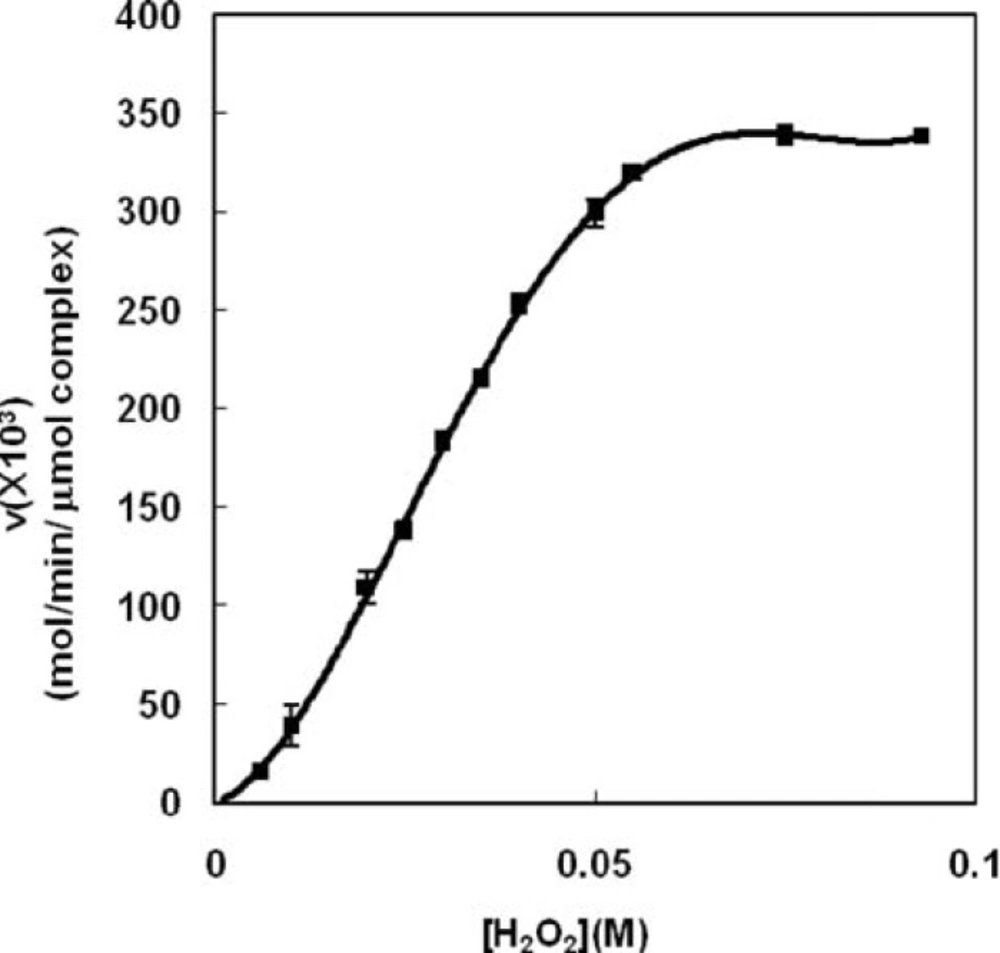
Because of the lack of kinetic studies on peroxidase activity of Haptoglobin-Hemoglobin complex, the present study has investigated kinetic properties of the complex enzymatic activity for the first time. The saturation curve of Hp (2-2)-Hb complex shows a sigmoid shape (Figure 1) indicating the activity of Hp (2-2)-Hb complex could not be analyzed using Michaelis-Menten model and requires the adoption of an enzyme model with multiple sites (33) showing cooperative binding for the substrate (H2O2).
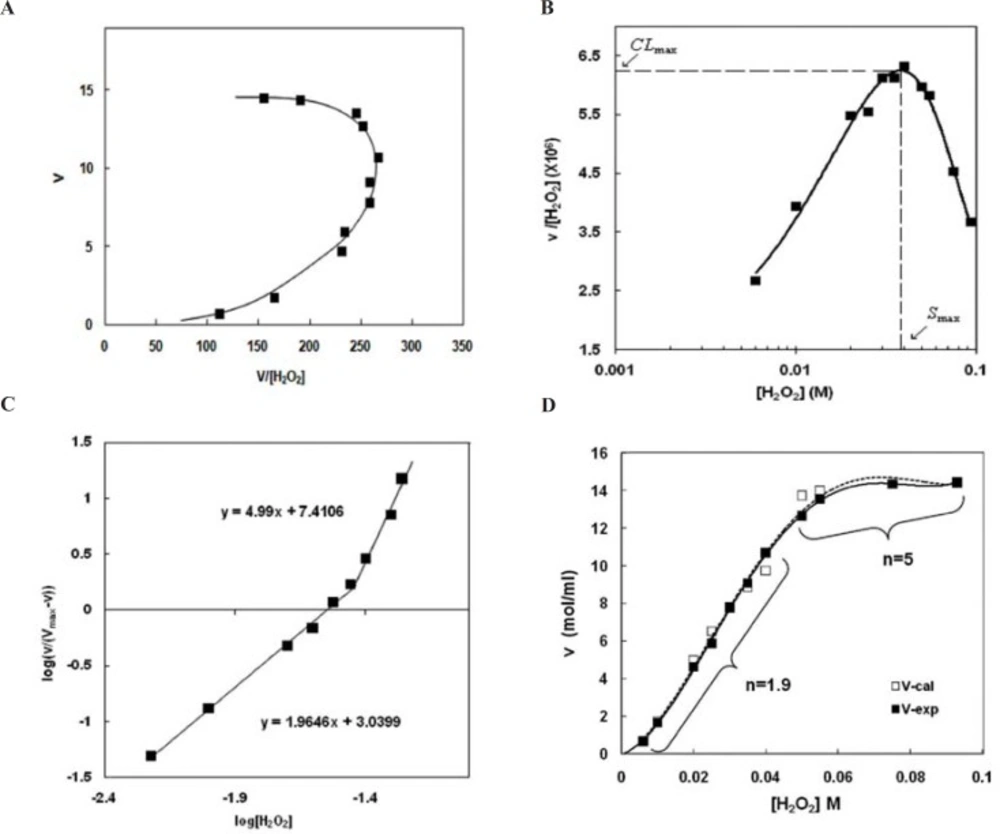
Eadie-Hofstee plot (Figure 2.A) confirms positive cooperativity deduced from the right-side curvature of the plot. This indicates that H2O2 not only is a substrate of the peroxidase activity, but also plays as an activator at higher concentrations of H2O2Figure 2.B shows that there is a well defined maximum for the clearance of the substrate, CLmax. It is worthy to note that CLmax is determined under in-vitro condition but it is appropriate parameter for investigation of the activity under in-vivo condition. The concentration of hydrogen peroxide that causes maximum clearance (CLmax) is named Smax. Both of the parameters were obtained from Clearance plot (Figure 2.B). Smax was observed in relatively low hydrogen peroxide concentrations, because of the maximum complex autoactivation. Indeed, CLmax provides an estimate of the highest clearance attained as substrate concentration increases before saturation of the enzyme sites.
Thus if the assumption is made that in-vivo activation occurs via endogenous activators, then CLmax may be an appropriate parameter for describing the salient feature of the subsystem that can be used for predictive purposes (33).
Hill plot(Figure 2.C) not only confirms positive allostric effect (Hill coefficients are more than 1, m>1), but also shows that there are two sequential positive allostric effects (m1=1.9, m2=5) with increasing H2O2 concentration (Table 1).
| m2 | m1 | S50 (M) | Smax (M) | CLmax (106) | Antibiotic |
|---|---|---|---|---|---|
| 5.0 | 1.9 | 0.03 | 0.04 | 6.3 | - |
| 2.8 | 1.9 | 0.03 | 0.04 | 7.3 | ampicillin |
| 7.8 | 1.9 | 0.03 | 0.05 | 6.8 | coamoxiclav |
Calculated initial velocities (V-cal) obtained from Hill equation using the Hill coefficients (see Material and Method) is in good coincidence with experimental initial velocity (V-exp) (Figure 2.D).
Non-Michaelis analysis of peroxidase activity of Hp (2-2)-Hb complex. A) Eadie-Hofstee plot. The nonlinear shape and the curvature toward right show non-Michaelis homotropic allostric effect. B) Clearance plot. As it is observed the upward curvature confirms homotropic property. Maximum clearance (CLmax) and Smax have been determined graphically as shown. C) Hill plot. It is seen the points of this graph lay on at least two consecutive linear parts. The slope of each line (m) is more than unit (m>1). This observation not only confirms positive cooperativity and homotropic effect but also demonstrates the behavior is changed with H2O2 concentrations so that the two sequential homotropic behaviors lead to increased activity. D) comparison between experimental and calculated saturation curve. Experimental initial velocities (-) are directly from figure 1. Calculated initial velocities (…..) have been calculated using Hill coefficients (Fig 2,C) and Hill equation as explained in Materials and Methods
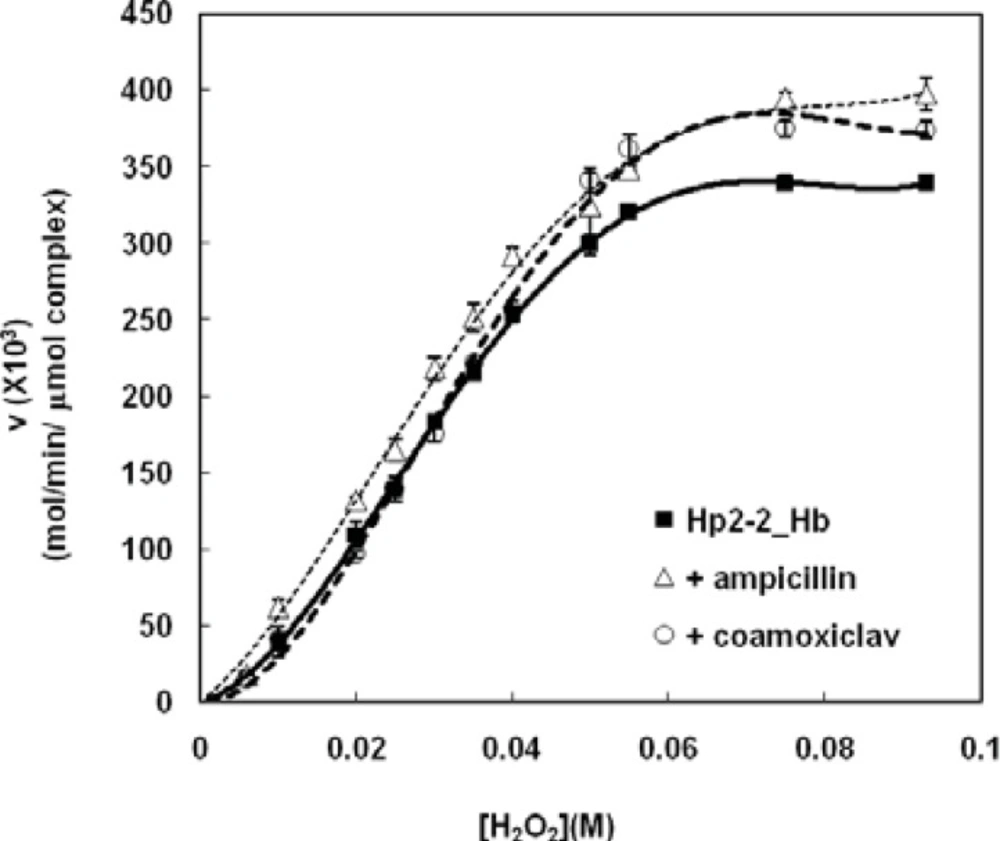
As it is shown in Figure 3; both ampicillin and coamoxiclav have activating effect on Hp (2-2)-Hb peroxidase activity (heterotropic effect or heteroactivation), but they have not changed the sigmoid shape of saturation curve (homotropic effect or autoactivation). It seems that both drugs induce a new conformation to the complex which is more active. In other word, autoactivation and heteroactivation have occurred simultaneously.
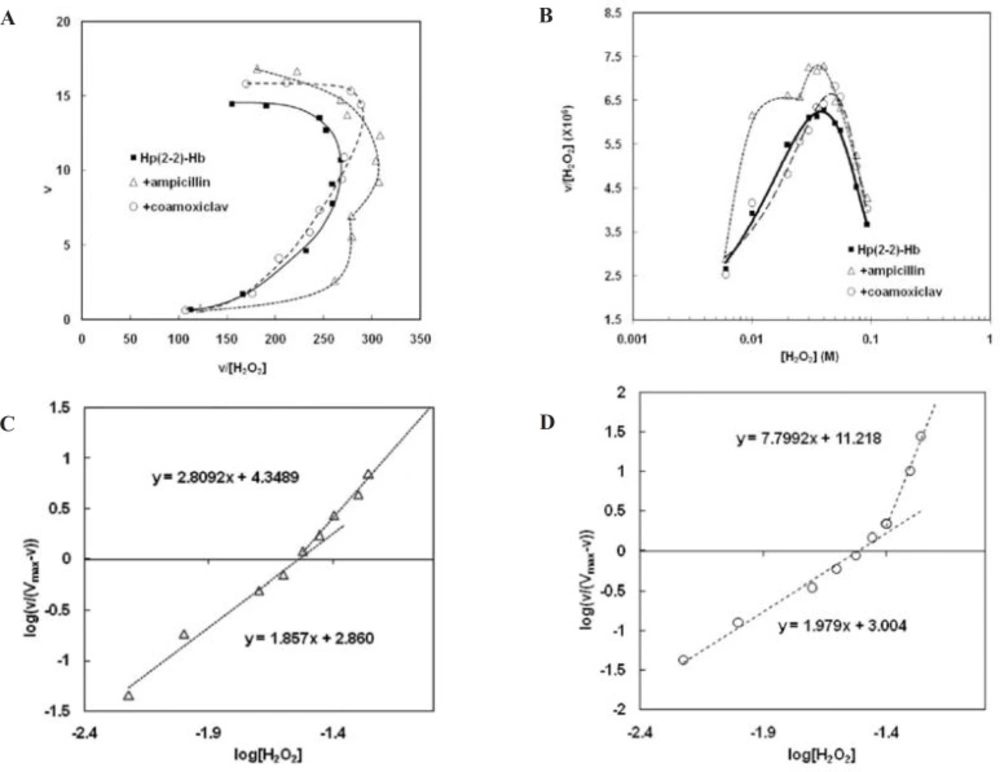
Analysis of the Hp (2-2)-Hb complexsaturation curves in the presence of the two antibiotics (Figure 4), has shown that Hp (2-2)-Hb complex maintains the homotropic behavior of its peroxidase activity. Moreover the drugs have not changed the first Hill coefficient, while the second Hill coefficient has been decreased in the presence of ampicillin and increased in the presence of coamoxiclav (Figure 4.C and 4.D, respectively). These results have been summarized in Table1. Comparison of the amounts of CLmax shows that both antibiotics increase CLmax of complex to remove H2O2 (Figure 4.B, Table1). This effect in the presence of ampicillin is two times more than of coamoxiclav (about 16% increasing in peroxidase activity in the presence of ampicillin and 8% in the presence of coamoxiclav). Smax has been shifted to relatively higher concentrations of H2O2 in thepresence of coamoxiclav, while ampicillin has not considerable effect on Smax (Figure 4.B, Table1). This indicate that maximum efficiency of Hp (2-2)-Hb complex for the clearance of H2O2 has been shifted to the higher concentrationsof H2O2. S50 has not been changed in the presence of the two drugs (Table1).
Non-Michaelis analysis of peroxidase activity of Hp (2-2)-Hb complex. A) Eadie-Hofstee plot. The nonlinear shape and the curvature toward right show non-Michaelis homotropic allostric effect. Eadie-Hofstee plot in the absence of drugs (Figure 2.A) has been incorporated here, for comparisoan.B) clearance plot. As it is observed the upward curcature confirms homotropic property. Maximum clearance (CLmax) and Smax have been determined graphically as shown. Clearance plot in the absence of drugs (Figure 2.B),has been incorporated here, for comparison. C) Hill plot in the presence of ampicillin. It is seen the points of this graph lay on at least two consecutive linear parts. The slope of each line (m) is more than unit (m>1). this observation not only confirms positive cooperativity and homotropic effect but also demonstrates the behavior is changed with H2O2 concentrations so that the two sequential homotropic behaviors lead to increased activity. D) Hill plot in the presence of coamoxiclav. The same effect can be observed here Moreover the slope of second line in the presence of coamoxiclav, is more than of ampicillin. the obtained values of CLmax, Smax and Hill coefficients have been summarized in Table1.
In conclusion, ampicillin and coamoxiclav can act as activators for peroxidase activity of Hp (2-2)-Hb complex via heterotropic effect. It will be meaningful if we remind that the two antibiotics are usually used under pathologic oxidative status caused by bacterial infections (19-21), and β-lactam antibiotics such as ampicillin have been shown to affect on their host oxidative status (15). This can be important in the individuals with phenotype Hp2-2 who have less antioxidant activity relative to other phenotypes and are susceptible to cardiovascular disorders (34). Therefore our in-vitro studies show that the two antibiotics (especially ampicillin) may help those individuals with Hp (2-2) phenotype to improve the Hp-Hb complex efficiency of removing hydrogen peroxide from serum under oxidative stress. This study needs to be continued by in-vivo experiments to confirm the conclusion under physiologic condition.