Introduction
Malaria is the most important problem of developing countries and is still an endemic disease in more than 100 countries (1). According to the latest report of World Health Organization, it kills between 1.5-2.7 million people every year (2). Malaria is endemic in the south of Iran (3) and has always been considered as the most important vector-borne disease in Iran due to its socioeconomic effects on the population (4). Since the discovery of the insecticide dichlorodiphenyltrichloroethane (DDT) before the Second World War, the wide-spread use of synthetic insecticides for the control of pests as well as human disease vectors has led to concerns about their toxicity and environmental impact (5). Because of this, the search for new environmentally safe, target-specific insecticides based on natural plant products, is active throughout the world.
The genus Centaurea L. (Asteraceae, tribe Cardueae, subtribe Centaureinae) comprises ca. 600 species distributed widely from Asia, Europe and Tropical Africa to North America as aggressively invading weeds (6). This genus consists of 88 species in the Flora Iranica (7). C. bruguierana (DC.) Hand.-Mzt. ssp. belangerana (DC.) Bornm. (Sect. Tetramorphaea) – a 5-50 cm annual herb with purple spiny flowers-is distributed in Iran, Transcaucasia, Afghanistan, Pakistan, and Central Asia (7).
Many species of the genus Centaurea have long been used in traditional medicines to cure various ailments, e.g. diabetes, diarrhea, rheumatism, malaria, and also against coughs, as liver-strengthening, itch-eliminating and ophthalmic remedies (8-10). Various biological activities have been reported for Centaurea spp. so far including antiviral and antimicrobial for C. solstitialis ssp. solstitialis (11), antibacterial for C. diffusa (12), antifungal for C. thessala ssp. drakiensis and C. attica (13), antiplasmodial for C. hierapolitana (14), C. eryngioides (15) and C. musimomum (16), cytotoxic for C. schischkinii (17), C. montana (8) and C. musimomum (16), anti-inflammatory, analgesic and antipyretic for C. ainetensis (18), C. chiliensis (19), C. tchihatcheffii (20), C. cyanus (21) and C. solstitialis ssp. solstitialis (22), anti-peptic ulcer and anti-Helicobacter pylori for C. solstitialis ssp. solstitialis (23-25). In addition, a variety of secondary metabolites have been reported from different species of this genus including sesquiterpene lactones (11, 26-28), flavonoids (8, 10, 17, 29, 30), lignans (8, 17, 26) and alkaloids (8, 17).
As a part of our ongoing larvicidal screening of native Iranian plants, in this paper, we describe for the first time the larvicidal activity of C. bruguierana ssp. belangerana against Anopheles stephensi Liston which is the main malaria vector in southern Iran and resistant to DDT, dieldrin and malathion in this area (31-32).
Experimental
Plant material
The whole flowering samples of C. bruguierana ssp. belangerana, namely “Baad-Avard”, were collected by “Agricultural Research and Natural Resources Center of Bushehr Province” from Borazjan (Borazjan, Bushehr Province) located in south of Iran, at an elevation of 70 m in June 2007 and identified by Professor Gh. Amin, Herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences (Tehran, Iran) where voucher specimen is deposited (6683-TEH).
Extraction and solvent-solvent fractionation
Dried whole flowering samples (300 g) were extracted with 80% methanol (MeOH, 6 × 1.5 l) in a percolator at room temperature for 2 weeks. The combined extract was concentrated to dryness under reduced pressure at 40°C. The MeOH extract was successively dissolved in 100 mL MeOH : H2O (7 : 3) and extracted with petroleum ether (4 × 200 mL), chloroform (CHCl3, 4 × 200 mL), H2O-saturated ethyl acetate (EtOAc, 4 × 200 mL) and H2O-saturated n-butanol (n-BuOH, 4 × 200 mL) in separatory funnel. Each fraction together with the remaining MeOH part after the solvent fractionation, were then evaporated to dryness under reduced pressure at 40°C for the purpose of test fraction. All solvents were purchased from Merck (Merck, Darmstadt, Germany).
Mosquitoes
Anopheles stephensi larvae used in this study were obtained from the laboratory of the “School of Public Health and Institute of Health Research” (Tehran University of Medical Sciences, Tehran, Iran) (originally from the malarious areas of Iran, Kazeroon, Fars province). They were reared under insectary conditions at 25 ± 1, 12/12 h (light/dark) photo-period and 50-70% relative humidity and were fed with 10% sucrose solution. The late 3rd and early 4th instar larvae were used for the tests. The sucrose solution was withdrawn from the cage, 14 h prior to the tests.
Larvicidal assay
The larvicidal activity of the total extract and fractions were assayed according to WHO methods (20). Preliminary testing was carried out to establish suitable stock solutions of the total extract and fractions as test samples. For each concentration, 4 replicates of 25 larvae were used. Each test run consisted of 224 mL water, 1 mL of test sample stock solution and 25 larvae in 25 mL water; so that the final volume was 250 mL. Finally, the resulted concentrations for test samples were as follows: 40, 20, 10, 5 and 2.5 ppm. In control runs, 1 mL of MeOH was added instead of test sample. Mortality was determined after a 24 h exposure period. In the analysis, both dead and moribund larvae were considered as dead. From the regression line between logarithmic dose and probit mortality, the LC50 was determined.
Statistical analysis
The percentage of mortality in the treated larvae was corrected relative to the control using Abbott’s formula (34). The mortality data were subjected to probit regression analysis according to Finney (35). The goodness of fit of the points to a straight line was tested by chi-square analysis. Data were computer analyzed through the probit plane procedure using MicroProbit software (version 3.0). From the regression line between the logarithmic dose and probit mortality, all the parameters including LC50, LC90, confidence interval (CI) and slope values were determined. Significant differences were determined through comparing the LC50 and 95% CI. The heterogeneity of the population was determined through the chi-square test. The regression line was plotted using Microsoft Excel.
Results and Discussion
The extraction of plant powder and the fractionation of extract yielded 32.0 g of the total extract, 0.976 g petroleum ether fraction, 4.268 g CHCl3 fraction, 3.394 g EtOAc fraction, 3.485 g n-BuOH fraction and 13.077 g of the remaining MeOH fraction.
The results of the bioassay tests of the total methanolic extract and fractions on the Anopheles stephensi larvae are presented in Table 1.
| (%) Mortality | Total dead | Test samplea |
|---|---|---|
| 20 | 28 | Total |
| 86 | 86 | Petroleum ether |
| 0 | 0 | Chloroform |
| 0 | 0 | Ethyl Acetate |
| 0 | 0 | n-Butanol |
| 0 | 0 | Remaining methanol |
| 0 | 0 | Control (Methanol) |
According to the mortality data, only the total extract and petroleum ether fraction had larvicidal activity with mortality rate of 28% and 86%, respectively, at concentration of 40 ppm, while the other fractions were inactive.
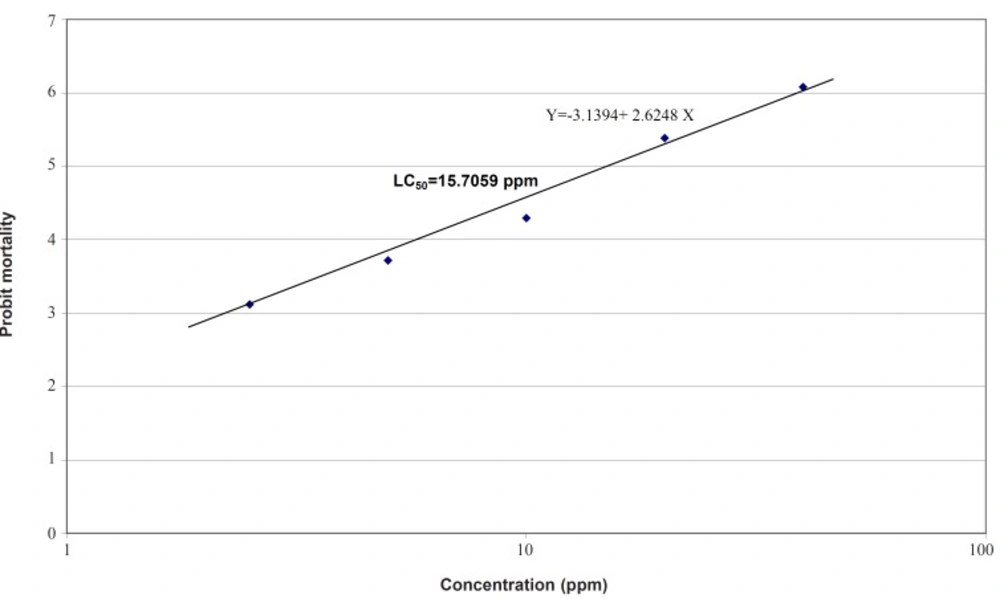
Therefore, the logarithmic concentrations were subjected to larvicidal assay for these two test samples (Tables 2 and 3). The probit regression line for petroleum ether fraction is plotted in Figure 1 and LC50, LC90, confidence interval (CI) and slope values are presented in Table 4. For petroleum ether fraction, the LC50 (lethal concentration to cause 50% mortality in population) and LC90 (lethal concentration to cause 90% mortality in population) were measured as 15.70 ppm and 48.3 ppm, respectively (Table 4).
| Mortality % | Total dead | Concentration ppm a |
|---|---|---|
| 28 | 28 | 40 |
| 12 | 12 | 20 |
| 4 | 4 | 10 |
| 0 | 0 | 5 |
| 0 | 0 | 2.5 |
| 0 | 0 | Control (Methanol) |
| Mortality (%) | Total dead | Concentration (ppm )a |
|---|---|---|
| 86 | 86 | 40 |
| 65 | 65 | 20 |
| 24 | 24 | 10 |
| 10 | 10 | 5 |
| 3 | 3 | 2.5 |
| 0 | 0 | Control (Methanol) |
| Intercept | Slope ± SE | LC50 (ppm)± 95% CI | LC90 (ppm)± 95% CI | χ2 | χ2table (df) | p-value |
|---|---|---|---|---|---|---|
| -3.1394 | 2.6248 ± 0.187 | 14.0035 | 40.3831 | 3.442* | 7.81 (3) | < 0.05 |
On the basis of the presence of nonpolar compounds in petroleum ether fraction, we can assume that the larvicidal activity of this fraction would be related to these compounds. On the other hand, using the biopesticides containing active nonpolar compounds would not produce water pollution because of their accumulation on the outer surface of the water where the larvae spread in swamps.