Introduction
The chronic inflammatory diseases are one of the major health problems worldwide and non-steroid anti-inflammatory drugs (NSAIDs) are the most prescribed drugs for the treatment of inflammatory diseases. Although the NSAIDs provide the patients with symptomatic relief, they do not modify the pathogenesis of inflammation and do not reduce the disabling bone and cartilage damage (1). Therefore, there has been substantial amount of research for finding pain relief medications and reducing the inflammation. The antinociceptive and anti-inflammatory effects of some plants such as Zataria multiflora (2, 3), Crocus sativus (4) Zhumeria majdae (5) and Elaeagnus angustifolia (6) Urtica pilulifera (7) Rosa damascena (8) have been reported previously, and the results proved good analgesic and anti-inflammatory activities.
Pistacia vera L., a member of Anacardiaceae family, has sedative and tonic effects (9). In folk medicine, it has been used as analgesic, carminative, astringent, stomachic, aphrodisiac, antitussive, diuretic, and expectorant (10). The kernels of P. vera are remarkably rich in linoleic and linolenic acids, which are the vital fatty acids for human health (11). In modern pharmacology, Pistacia species have been reported to have various biological effects such as antiatherogenic, hypoglycemic, antioxidant, antiprotozoal, analgesic and anti-inflammatory (12-13). The aqueous extract of leaves and nuts of P. vera has antiemetic activity in young chickens with peripheral and central mechanisms (14). In a study, P. vera gum exhibited neuroprotective effects against ischemia (14). In different studies, P. weinmannifolia showed a potent antioxidant activity (16-17). The ethanolic extract of P. vera gum has a protective activity against liver damage induced by CCL4 in rats (16), and in another study, ethanolic extract of P. vera gum showed antinociceptive effect (19).
The chemical constituents of the Pistacia genus were studied, and monoterpenes (18), tetracyclic triterpenoids (21), flavonoids (22), and other phenolics including gallic acid (16-23) and essential oils (24) were found. Six gallotannins and seven flavonoid glycosides were isolated from P. weinmannifolia (25). P. terebinthus has acute and chronic anti-inflammatory effects, and its main ingredient, oleanonic acid which is a 3-Oxotriterpenoid, has leukotriene anti-synthetic property (12). In a study, P. anacardiaceae showed a noticeable analgesic and anti-inflammatory effect at in-vivo tests (26). Since I.R. Iran is one of the main origins of pistachio and among the species of Pistacia genus, P. vera is the most economically important one, we investigate the anti-inflammatory and analgesic effect of this species.
Experimental
Plant material
The plant was collected from south of Khorasan (Gonabad, Iran), dried in shadow, and ground. The voucher samples were preserved for reference in the Herbarium of Pharmacognosy Department, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran (with Voucher No. of 165-2502-2). The plant powder was extracted using aqueous infusion and maceration with ethanol. For the aqueous infusion, hot water was added to plant material (100 g) and boiled for 15 min, then filtered through a clean cloth. For ethanolic extraction, the plant powder was macerated in ethanol (80%, v/v) for 72 h and the mixture was subsequently filtered (5, 27).
Animals
Male and female albino mice of 25-30 g were used. Animals were obtained from Razi Institute and Pharmacology Lab in Mashhad University of Medical Science and were housed under a 12/12 h light/dark cycle at 21 +/- 2°C with free access to water and food. The handling and use of animals were in accordance to the institutional guidelines and all experiments were carried out in accordance with current and ethical guidelines for the care and use of laboratory animals.
Acute toxicity tests
Different doses of extracts were injected into groups of six mice intraperitoneally (IP) and the number of deaths was calculated at 48 h after treatment. LD50 values and corresponding confidence limits were determined using the Litchfield-Wilcoxon method (PHARM/PCS Version 4).
Antinociceptive study
Hot plate test
Evaluation of antinociceptive activities of aqueous and ethanolic extracts
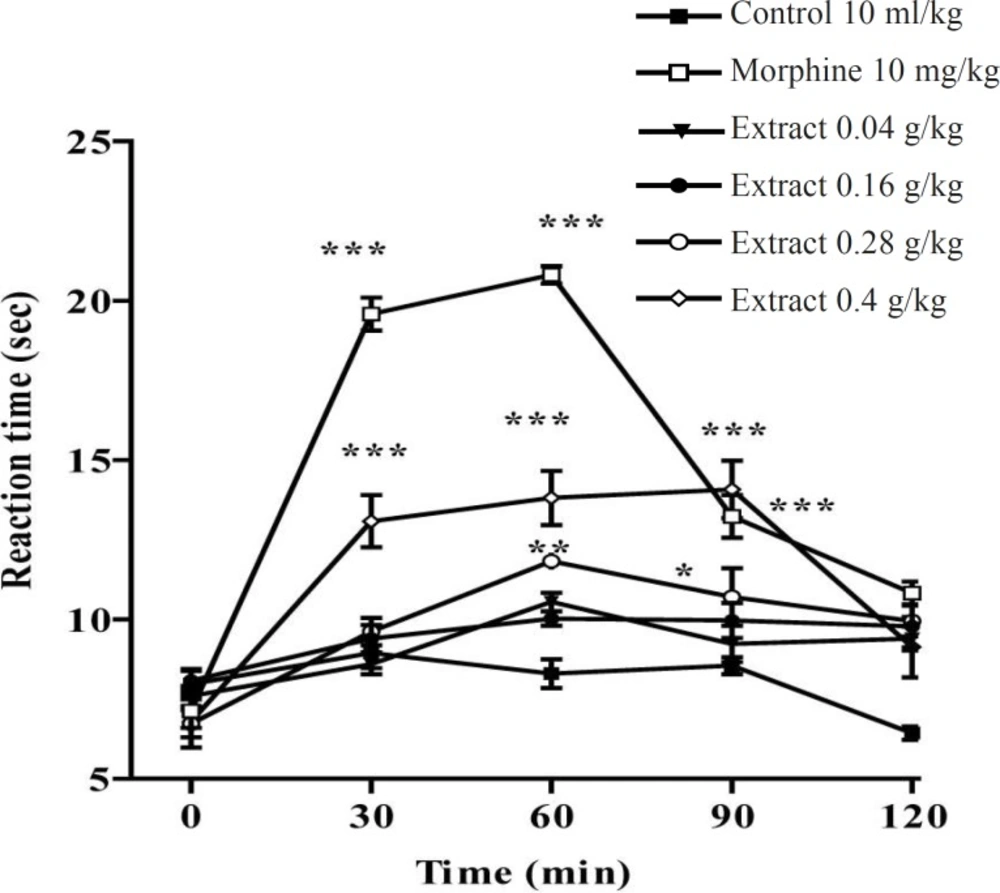
According to the results of acute toxicity test, the highest doses without mortality for aqueous and ethanolic extracts were 0.4 g/Kg and 0.5 g/Kg respectively. We chose 4 groups of doses based on 100, 70, 40 and 10% of the highest dose for aqueous (0.04, 0.16, 0.28, 0.4 g/Kg) and ethanolic extracts (0.05, 0.2, 0.35, 0.5 g/Kg). We used saline and morphine (10 mg/Kg IP) as negative and positive controls.
In hot plate test, the aqueous and ethanolic extracts were evaluated in six groups of 6 mice. The temperature of the metal surface was 55°C and the latency to a discomfort reaction (jumping or licking paws) was measured. The cut-off time was 40 sec (28). The responses were recorded in 0, 30, 60, 90 and 120 min after administration.
Effect of naloxone on antinociceptive activity of aqueous and ethanolic extracts
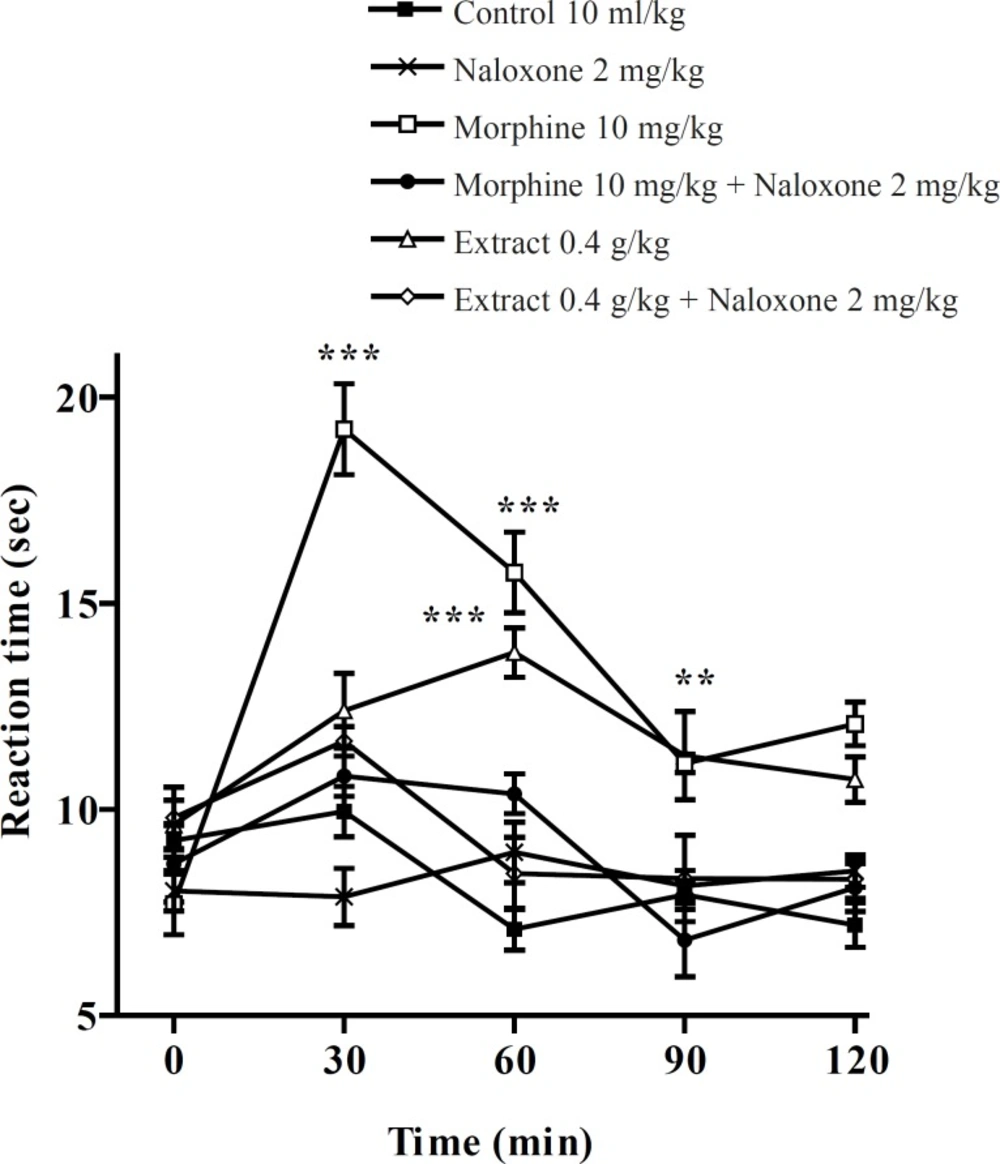
According to the previous test and its results, we chose the doses of 0.4 g/Kg for aqueous and 0.35, 0.5 g/Kg for ethanolic extracts. Two separate tests were performed (6 groups for aqueous and 8 groups for ethanolic extracts). Pretreatment with Naloxone (2 mg/Kg SC) was carried out 15 min before the administration of a morphine group (10 mg/Kg IP) and one group for each extract. We also used each extract separately and normal saline (10 mg/Kg) and morphine (10 mg/Kg) as negative and positive controls.
Writhing test
The aqueous and ethanolic extracts were administered to the groups of 6 mice in two doses, and 30 min later an intraperitoneal injection of 0.7% v/v acetic acid solution were given to mice (0.1 mL/10 g). The number of writhes was counted for 30 min (29).
Anti-inflammatory study
Xylene-induced ear edema
Mice were divided into 5 groups of six. Sixty min after the IP injection of the extracts or diclofenac (as positive control) or normal saline (as negative control), one drop of xylene was applied to the anterior surfaces of the left ear and the right ear was considered as a control. Two h after the Xylene application, animals were killed and both ears were removed. Circular sections were applied (4.5 mm) and weighed. The difference of weight in the left ear section from that of the right was considered as edema (30).
Cotton pellet granuloma
The chronic anti-inflammatory activity was tested using granuloma pouch method. We used 4 groups of mice: 2 groups for extracts, one normal saline as negative and diclofenac as positive controls. Sterile cotton gauze dental packs were washed with a solution of Cetrimide-C. After total anesthesia, dorsal shoulders of mice were shaved and disinfected. Two cotton pellets impregnated with 0.4 mL of an aqueous solution of ampicillin were implanted in shoulders of mice (SC), one on each side. In the following seven days, the animals were treated with extracts or diclofenac. On day 8, the mice were killed and the pellets and surrounding granulation tissues were dried at 60°C for 24 h. The weight of granuloma and the percentage inhibition of inflammation were measured for all the groups (30).
Statistical analysis
Statistical analysis was carried out using one-way ANOVA. Level of p < 0.05 was considered as significant. The data were reported as mean ± SEM and tested with analysis of variance followed by the multiple comparison test of Tukey-Kramer.
Results and Discussion
The LD50 values of the infusion and maceration extracts were 0.8 g/Kg and 0.79 g/Kg, respectively and the maximum non-fatal doses were 0.4 g/Kg and 0.5 g/Kg respectively.
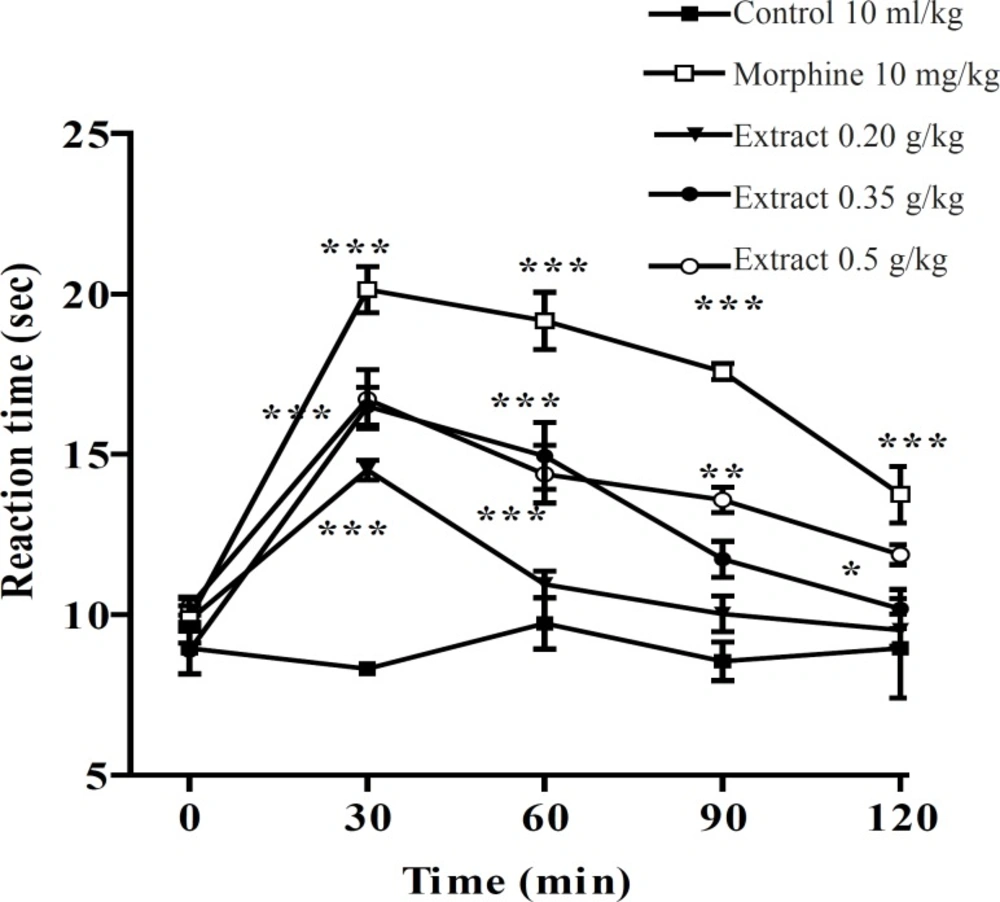
In the hot plate test, the administration of the aqueous and ethanolic extract demonstrated a dose-dependent antinociceptive activity after 30-60 min of treatment (Figures 1 and 2).
Effect of the ethanolic extract of Pistacia vera leaf extract and morphine (IP) on the pain threshold of mice in the hot plate test. Each point represents the mean ± SEM. of reaction time for n = 6 experiments on mice, compared with control; *: p < 0.05; **: p < 0.01; ***: p < 0.001; Tukey-Kramer test
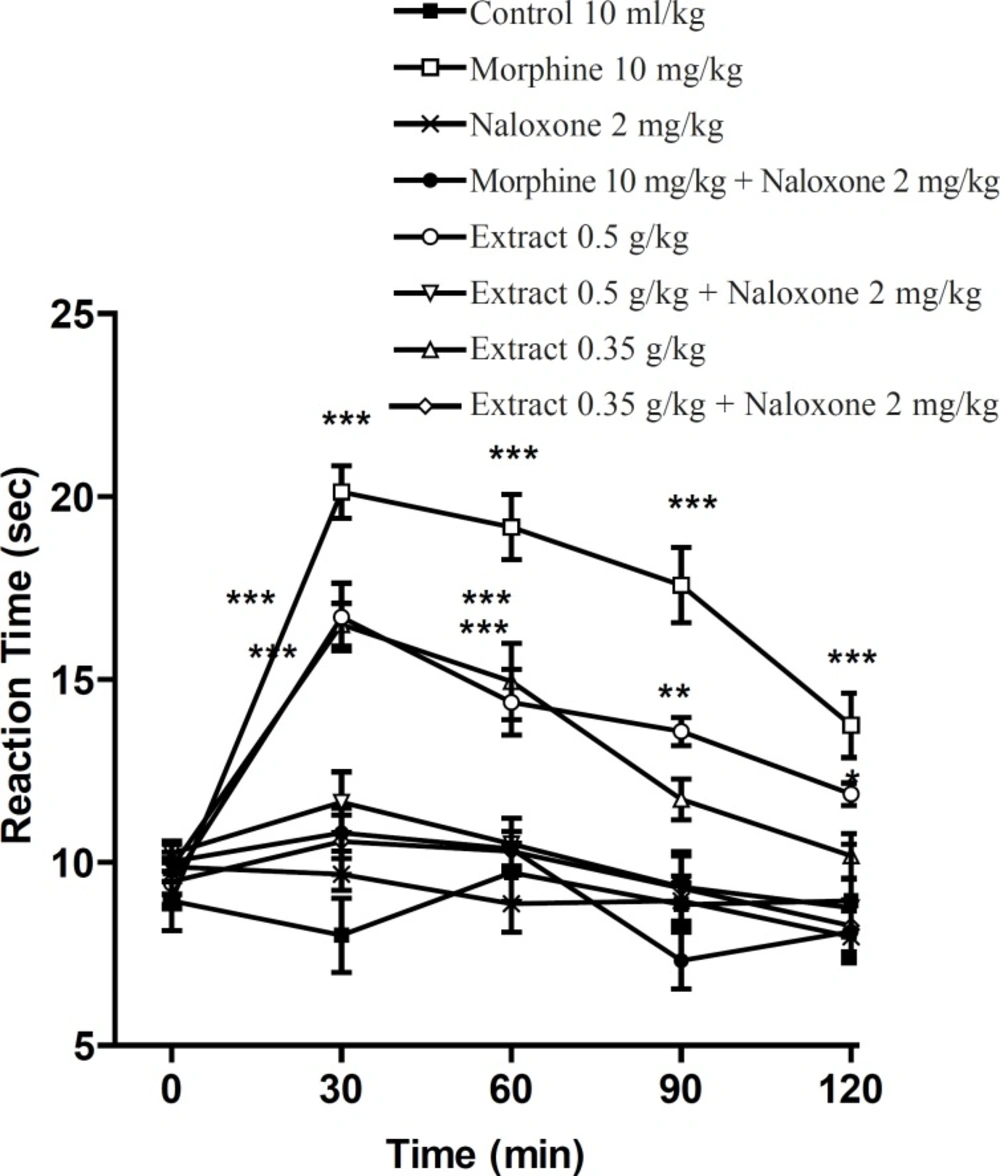
Pretreatment with naloxone (2 mg/Kg, IP) inhibited the antinociceptive activity of the aqueous extract (0.4 g/Kg, IP) and morphine (10 mg/Kg, IP), sixty min after the injection. Only at the time of 60 min, extract (0.4 g/Kg) was more effective than normal saline and the antinociceptive effect showed a plateau effect between 30 to 90 min. The ethanolic extract also showed the dose-dependent antinociceptive activity that was inhibited by naloxone (Figures 3 and 4).
Effect of naloxone (SC) on the ethanolic extract of Pistacia vera leaf and morphine antinociceptive activity in mice (hot plate test). Each point represents the mean ± SEM of reaction time for n = 6 experiments on mice, compared with control, *: p < 0.05; **: p < 0.01; ***: p < 0.001; Tukey-Kramer test
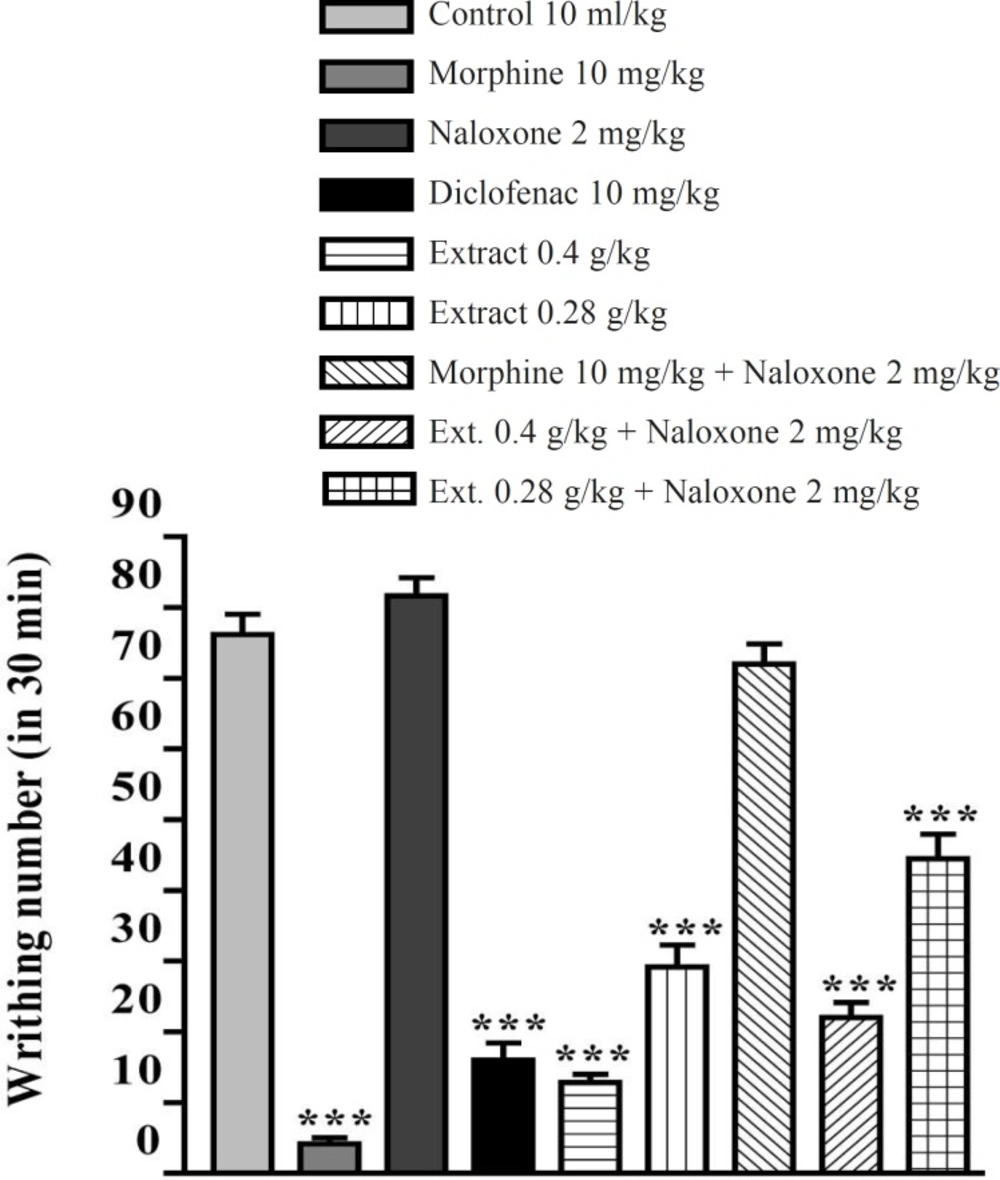
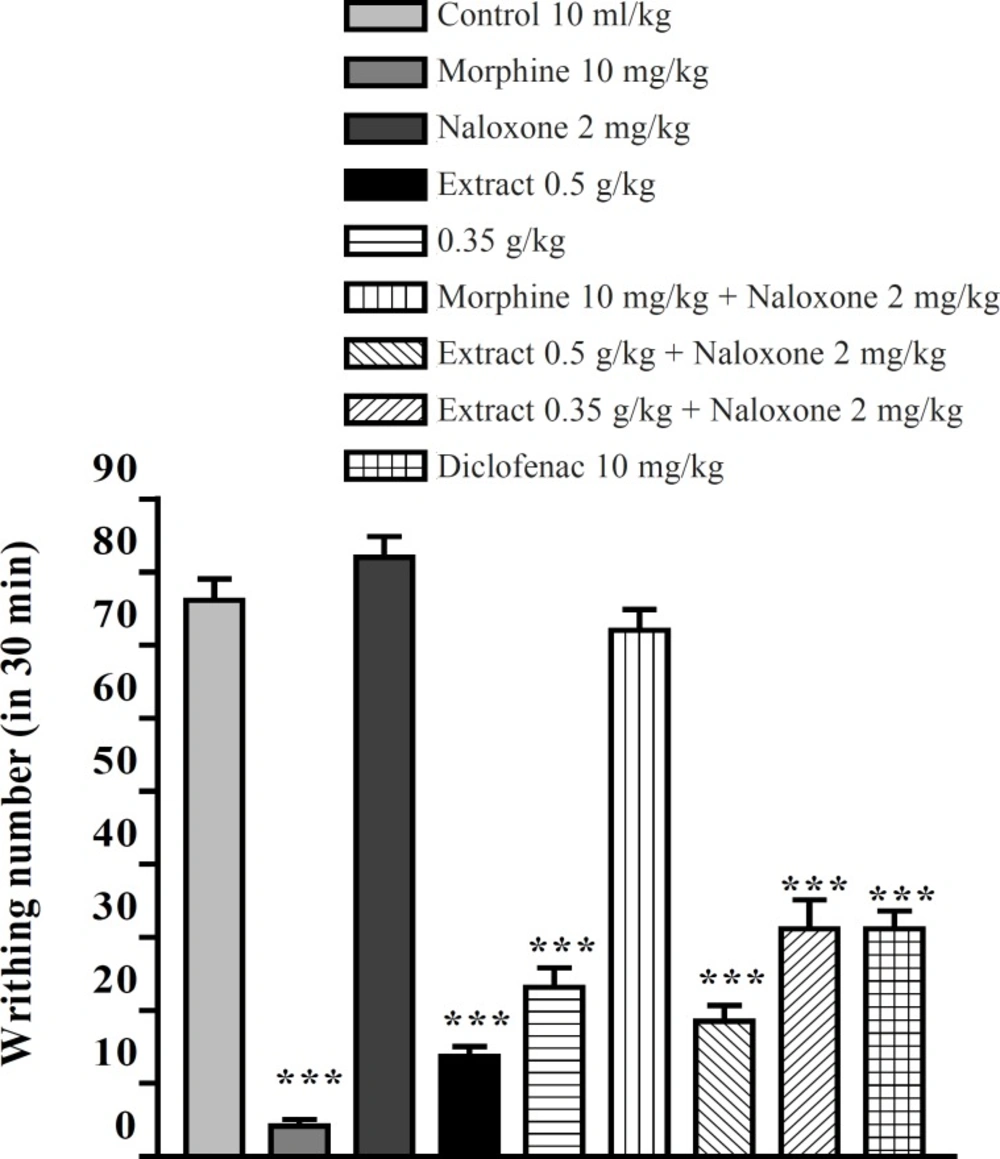
The aqueous and ethanolic extracts of P. vera leaves significantly reduced the number of mouse abdominal constrictions induced by an acetic acid solution. There was no significant difference between diclofenac (10 mg/Kg), morphine (10 mg/Kg) and the extracts in reduction of writhing numbers. Pretreatment with naloxone (2 mg/Kg IP) after the IP injection of extracts could not inhibit the antinociceptive activity of both extracts (Figures 5 and 6). In the xylene-induced ear edema study, the highest doses of aqueous and ethanolic extracts showed significant anti-inflammatory activities with %56 and %66 of inflammation and inhibition after 1 h, respectively. The ethanolic extract showed higher activity against the acute inflammation than did the aqueous extract (Tables 1 and 2).
| Treatment | Dose | Ear swelling (mg) | Inhibition (%) |
|---|---|---|---|
| Normal saline | 10 mg/Kg | 3.8 ± 0.3 | - |
| Diclofenac | 15 mg/Kg | 1.3 ± 0.3*** | 66.31 |
| Aqueous extract | 0.4 g/Kg | 1.6 ± 0.3 ** | 56.76 |
| Aqueous extract | 0.28 g/Kg | 2.8 ± 0.3* | 44.74 |
| Aqueous extract | 0.16 g/Kg | 2.8 ± 0.4 | 26.25 |
Effect of the intraperitoneal doses of Pistacia vera aqueous leaf extracts on Xylene-induced ear swelling in mice
| Treatment | Dose | Ear swelling (mg) | Inhibition (%) |
|---|---|---|---|
| Normal saline | 10 mg/Kg | 4.6 ± 0.4 | - |
| Diclofenac | 15 mg/Kg | 2.0 ± 0.4* | 55.86 |
| Ethanolic Extract | 0.5 g/Kg | 1.5 ± 0.3 ** | 66.95 |
| Ethanolic Extract | 0.35 g/Kg | 4 ± 0.7 | 12.47 |
Effect of the intraperitoneal doses of Pistacia vera ethanolic leaf extracts on Xylene-induced ear swelling in mice
In the chronic inflammation (cotton-plate) test, both extracts showed significant and dose-dependent anti-inflammatory activity. A dose of 0.5 g/Kg of ethanolic extract, showed a higher effect than diclofenac 15 mg/Kg (Tables 3 and 4).
| Treatment | Dose | Cotton pellet (mg) | Inhibition (%) |
|---|---|---|---|
| Normal saline | 10 mL/Kg | 11.0 ± 0.8 | - |
| Diclofenac | 15 mg/Kg | 4.0 ± 0.3*** | 64.12 |
| Aqueous Extract | 0.4 g/Kg | 4.3 ± 0.5*** | 61.11 |
| Aqueous Extract | 0.28 g/Kg | 5.1 ± 0.5*** | 53.31 |
Effect of the intraperitoneal doses of Pistacia vera aqueous leaf extracts (consecutive for 7 days) on the weight of granuloma in mice
| Treatment | Dose | Cotton pellet (mg) | Inhibition (%) |
|---|---|---|---|
| Normal saline | 10 mL/Kg | 11.0 ± 0.8 | - |
| Diclofenac | 15 mg/Kg | 4.0 ± 0.3*** | 64.12 |
| Ethanolic Extract | 0.5 g/Kg | 3.5 ± 0.5*** | 68.48 |
| Ethanolic Extract | 0.35 g/Kg | 5.5 ± 0.7*** | 50.04 |
Effect of the intraperitoneal doses of Pistacia vera ethanolic leaf extracts (for 7 consecutive days) on the weight of granuloma in mice
The present results showed that the aqueous and ethanolic extracts of P. vera leave have peripheral and central antinociceptive activity. The extract also demonstrated anti-inflammatory effects against acute and chronic inflammation.
The ethanolic extract showed more analgesic activity than aqueous type. Different studies showed that most of the ingredients of Pistacia species are oil-soluble. Ansari shows 3 triterpenes as major components of P. integerrima (31). One of the most identified active triterpenoids is oleanolic acid, which was acquired from P. terebinthus (10). In addition, oleoresin was shown to possess a marked anti-inflammatory activity (26). α-Pinene, a monoterpene-type of compound, is the dominant component in the essential oils obtained from leaves of P. vera (29.2%) and resin of P. lentiscus (21.7%) (32). α-Pinene showed anti-inflammatory effect (31). We can conclude that these oily-soluble components are the important cause of antinociceptive and anti-inflammatory effects of P. vera leaves.
With respect to the LD50 value and in comparison with a toxicity classification (34), the extracts were semi-toxic.
The aqueous and ethanolic extracts showed antinociceptive activity in the hot plate test and this effect was inhibited by naloxone. The hot plate test is a specific central antinociceptive test (35). Thus, it is suggested that the antinociceptive activity part of P. vera leaf may be mediated through a morphine-like and central activity.
As the preliminary phytochemical results indicated, the antinociceptive and anti-inflammatory effects of extracts may be due to their content of triterpenoid tetracycline. Other studies have demonstrated that triterpenes have significant antinociceptive and anti-inflammatory activities (31).
The antinociceptive activity of opioid agonist and non-steroidal anti-inflammatory agents can be determined using the writhing test (36). The antinociceptive activity of the extracts was not inhibited by naloxone completely, so other mechanisms of action, such as cyclooxygenase inhibition and peripheral mechanism may be considered.
In Xylene-induced ear edema test, increasing the vascular permeability caused edema in tissue. Following the stimulation by Xylene, mediators are released and induce the dilation of arterioles and venules. We can suggest that P. vera may have inhibitory effects on the release of mediators and also has a membrane-stabilizing effect that reduces capillary permeability.
The extract also reduced cotton pellet-induced granuloma, thereby suggesting its activity in the proliferative phase of the inflammation. It is concluded that extracts shows anti-inflammatory effect by inhibition of mediators› synthesis or other mechanisms like steroid factors.
A study on volatile essence of 3 Pistacia species showed that hydrocarbon monoterpenes were the main part. Oleanic acid is one of the best known biologically active triterpenoid (a 3-oxotriterpenoid), which has anti-inflammation and leukotriene inhibition synthesis effects (12).
It is concluded that the aqueous and ethanolic extracts of P. vera leaf have peripheral and central analgesic effects. Opioid receptors and the inhibition of cyclooxygenase enzyme may mediate these activities. The extracts also have activity against acute and especially chronic inflammation. The chemical constituents responsible for the pharmacological activities remain to be investigated.