Introduction
Pogostemon parviflorus has a strong odor. It grows in areas with high annual rainfall (Figures 1 and 2). This plant has antiseptic activity and it is useful in the treatment of enteritis, eczema and mycotic enteritis (1). Due to the increasing number of immunocompromised individuals, fungal infections have increased in the last two decades (2), among which skin fungal infections are very difficult to eradicate (3). Dermatophytes produce a variety of problems, such as Athlete’s foot and nail infections which lead to debilitation of the patients, and they can also spread to other areas of the body and to other individuals (4). Human mycoses are not always successfully treated because of their resistance to antifungal drugs, or ineffectiveness and side effects of these agents. Hence, it is of urgent importance to search for more effective and less toxic new antifungal agents through their detection in medicinal plants. Furthermore, new antifungal agents are still needed to improve the treatment of superficial fungal infections (5, 6).
Experimental
Plant material
Pogostemon parviflorus was collected from Mulshi district, Pune Maharashtra state, India.
This plant specimen was collected in flowering and fruiting state and identified by the botanical survey of India. A voucher specimen has been deposited at the herbarium of Botany department of Pune University, India. The healthy and disease-free leaves were separated and shade dried to avoid decomposition of chemical constituents. Leaves were powdered in grinder and stored in clean and dry airtight containers for further studies.
Preparation of plant extracts
The leaves of Pogostemon parviflorus were extracted in ethanol. To 10 g of each powdered material was added 100 mL ethanol 80% (drug/solvent ratio of 1:10 w/v) in a conical flask for maceration.Flask was then plugged with cotton and placed on a rotary shaker at 190-220 rpm for 72 h at room temperature (7). Finally, the suspension was filtrated through a Buckner funnel and Whatman filter paper #1.
The ethanolic extract was evaporated to dryness in an oven or in a water bath at 45°C. One gram of the dried extract was then dissolved in 1 mL 100 % dimethyl sulfoxide (DMSO). The final concentration of each extract was adjusted to 1000 mg/mL.
Dermatophyte isolates
For the evaluation of antifungal activity, 3 strains obtained from the Persian Type Culture Collection (PTCC), Tehran, Iran, including Trichophyton mentagrophytes PTCC 5054, Microsporum canis PTCC 5069, M. gypseum PTCC 5070; 13 as well as other strains isolated from different lesions of patients in a clinical laboratory in Ahwaz, Iran, including Microsporum canis (n = 2): MC-1, MC-2, M. gypseum (n = 3): MG-1, MG-2, MG-3, Trichophyton rubrum (n = 2): TR-1, TR-2, T. mentagrophytes (n = 3): TM-1, TM-2, TM-3 and Epidermophyton floccosum (n = 3): EF-1, EF-2, EF-3 and identified by standard procedures (8). Sabouraud dextrose agar (SDA) at 25°C was used to maintain isolates. For antifungal assay, each dermatophyte isolate was subcultured onto sabouraud dextrose agar (Hi-Media, India) slants and incubated at 28-30°C for 4-5 days and subcultured every 15 days to prevent pleomorphic transformations (7).
Preparation of fungal inoculum
A standardized inoculum was prepared by counting the microconidia, microscopically. For this purpose, the suspension of conidia was prepared using sterile distilled water or 0.85% physiological saline solution. The dispersing fluid was added to the slant tube culture and the surface of culture was gently rubbed by a sterile bent glass rod to dislodge the conidia from the hyphal mat. The suspension was then transferred to a sterile centrifuge tube and the volume was adjusted to 5-10 mL with sterile saline. The final suspension of conidia was counted with a hemocytometer cell counting chamber. The inoculum of cell or spore suspensions were prepared, as described elsewhere (9, 10) , and adjusted to 104-105 colony-forming units (CFU) per mL.
Antifungal susceptibility testing
The fungistatic activity of different extracts was evaluated by the agar dilution method (7, 11, 12). One thousand milligrams of the ethanolic extract was dissolved in 1 mL of sterile DMSO, serving as the stock solution (7). For the assay method, the stock solution of extract was two-fold diluted with sterile distilled water or saline solution to produce serial decreasing dilutions ranging from 0.078-20 mg/mL. Then 5 mL Mycosel agar medium was dispensed in each petri dish (60 mm in diameter), under laminar flow (aseptic condition), and cooled to 45°C. Into the non-solidified media 100 μL of the extract stock solution plus 50 μL of the dermatophyte suspension (105 CUF/mL) removed from a seven days old culture of fungi, was added, and evenly mixed. The plates were then incubated at 28-30°C.
MICs were visually recorded, based on the control fungus growth, up to 15 days for dermatophytic species. The antifungal agents like griseofulvin (Sigma) and Ketoconazole (Janssen Pharmaceutical) were used as the positive controls. Drug-free solution (only containing an appropriate amount of DMSO) was also used as the blank control for verification of fungal growth. MIC value was defined as the lowest extract concentration capable of inhibiting fungal growth, and MFC value was defined as the lowest extract concentrations showing no visible fungal growth after the incubation time. MIC50 and MIC90 values were the lowest extract concentrations at which 50% and 90% of the clinical isolates were inhibited (13). Dermatophyte plates were examined visually for 50% and 90% growth inhibition, compared to the growth control. MIC results were recorded in μg/mL. Every strain was tested in triplicate and a new inoculum was prepared for each assay. Duplicate plates were used for each assay.
Phytochemical study
The leaf of Pogostemon parviflorus was evaluated qualitatively for the presence of saponins, reducing sugars, tannins, alkaloids, proteins, glycosides, anthraquinones and flavonoids. In the present investigation, the ethyl acetate extract of Pogostemon parviflorus leaf was subjected to TLC, using a precoated silica gel F254 plate. A solvent system of acetone . ethyl acetate . petroleum ether (0.5: 0.5:2.0) [AEP] was used for obtaining the best resolution for spots. HPTLC fingerprint for the same extract was obtained at 254 nm and 366 nm.
Statistical analysis
Data analysis was performed, using the SPSS program version 10 (SPSS Inc., USA). Analysis of variance was conducted, using the general one-way ANOVA with post hoc comparison of mean values by LSD.
Results and Discussion
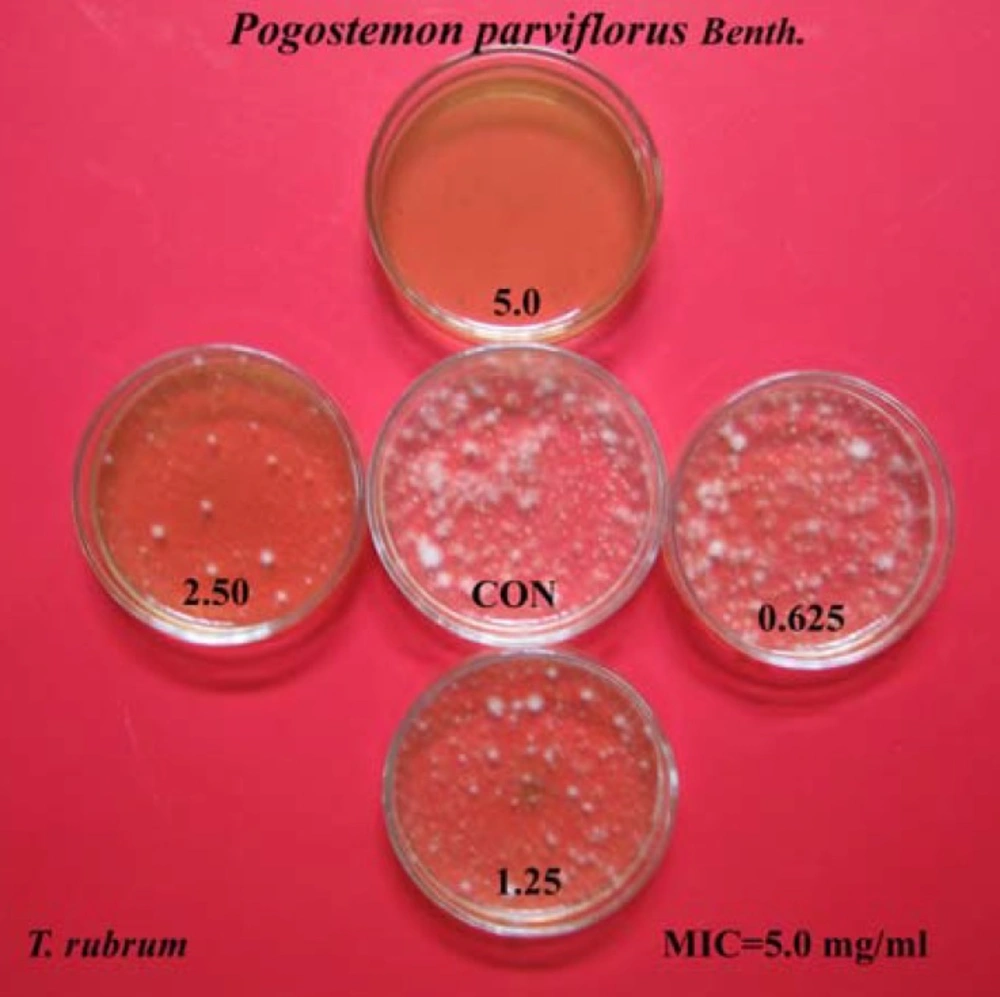
The ethanolic extract of Pogostemon parviflorus leaf completely prevented the growth of tested dermatophytic species, with MIC values between 2.5-10 mg/mL. MIC90 and MIC50 values were 1.250-5.000 and 0.312-1.250 mg/mL, respectively. The lowest MIC90s and MIC50s were concerned with the species of T. mentagrophytes and the highest MIC90s and MIC50s were observed with the strains of M. canis. The MFC values of this plant were also in the range of 2.5-10 mg/mL. Finally, the T. mentagrophytes species were found to be more sensitive than the other dermatophytic species, while species of M. canis were the most resistant among the five tested dermatophytic species, against inhibitory effects of Pogostemon parviflorus. The results have been shown in Tables 1 and 2, as well as Figure 3.
| Test agent | MIC value (mg/mL)a | |||||
|---|---|---|---|---|---|---|
| Mcb | Mgb | Efb | Trb | Tmb | ||
| Pogostemon parviflorus | 10.00 | 5.00 | 5.00 | 5.00 | 2.500 | |
| Griseofulvinc | 12.5 | 100 | 25 | 50 | 100 | |
| Ketoconazolec | 25.00 | 6.25 | 0.78 | 25.00 | 6.25 | |
MICs (mg/mL) of Pogostemon parviflorus leaf extract, compared to griseofulvin and Ketoconazole.
| Dermatophytes | Antifungal Compounds | MIC a and MFC | ||||
|---|---|---|---|---|---|---|
| Range | 50%b | 90%b | MFC | Geometric mean MIC | ||
| T. mentagrophytes (3) | KTZ GRS PPL | 0.78-6.25 | 1.56 | 6.25 | 12.5 | 3.52 |
| M. gypseum (3) | KTZ GRS PPL | 0. 78-6.25 | 1.56 | 6.25 | 12.5 | 3.52 |
| M. canis (3) | KTZ GRS PPL | 1.56- 12.50 | 3.12 | 12.5 | 25 | 7.03 |
| T. rubrum (2) | KTZ GRS PPL | 3.12-25.00 | 6.25 | 25 | 50 | 14.06 |
| E. floccosum (3) | KTZ GRS PPL | 0.39- 0.78 | 0.39 | 0.78 | 1.56 | 0.585 |
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of Pogostemon parviflorus leaf extract, griseofulvin and Ketoconazole against dermatophytes, using the agar dilution method
We also tested Pogostemon parviflorus against several strains of E. floccosum that produces arthroconidia. These microorganisms survive for a longer time than the other dermatophytes, and as a result constitute an environmental source of contagion, sometimes leading to recurrent outbreaks of dermatophytosis (6). Trichophyton rubrum and T. mentagrophytes, which are the main cause of athlete’s foot and onichomycoses in human beings, were also tested. Athlete’s foot is the most prevalent superficial infection in the developed world (14) and onichomycoses affects 2%–13% of the population worldwide and up to 30% of groups at high risk, such as elderly and diabetic people (15, 16).
Results of phytochemical screening indicated that the leaf of Pogostemon parviflorus contained saponins, reducing sugars, tannins, phenols and proteins, but not glycosides, anthraquinones, alkaloids and flavonoids (Table 3).
| Name of the test carried out | Reagents used | End result |
|---|---|---|
| A. Water extract | ||
| Starch | I2-KI | + |
| Tannins | Acidic FeCl3 | + |
| Saponins | H2SO4 + Acetic unhydride | + |
| Proteins | Millon’s test | + |
| Anthraquinones | + Benzene | - |
| Reducing sugars | Benedict’s | + |
| B. Alcoholic extract | ||
| Alkaloids | Mayer’s | - |
| Wagner’s | - | |
| Dragendorff’s | - | |
| Flavonoids | HCl + Mg turnings | - |
| Glycosides | Benzene+hot ethanol | - |
Phytochemical screening of Pogostemon parviflorus leaf extracts
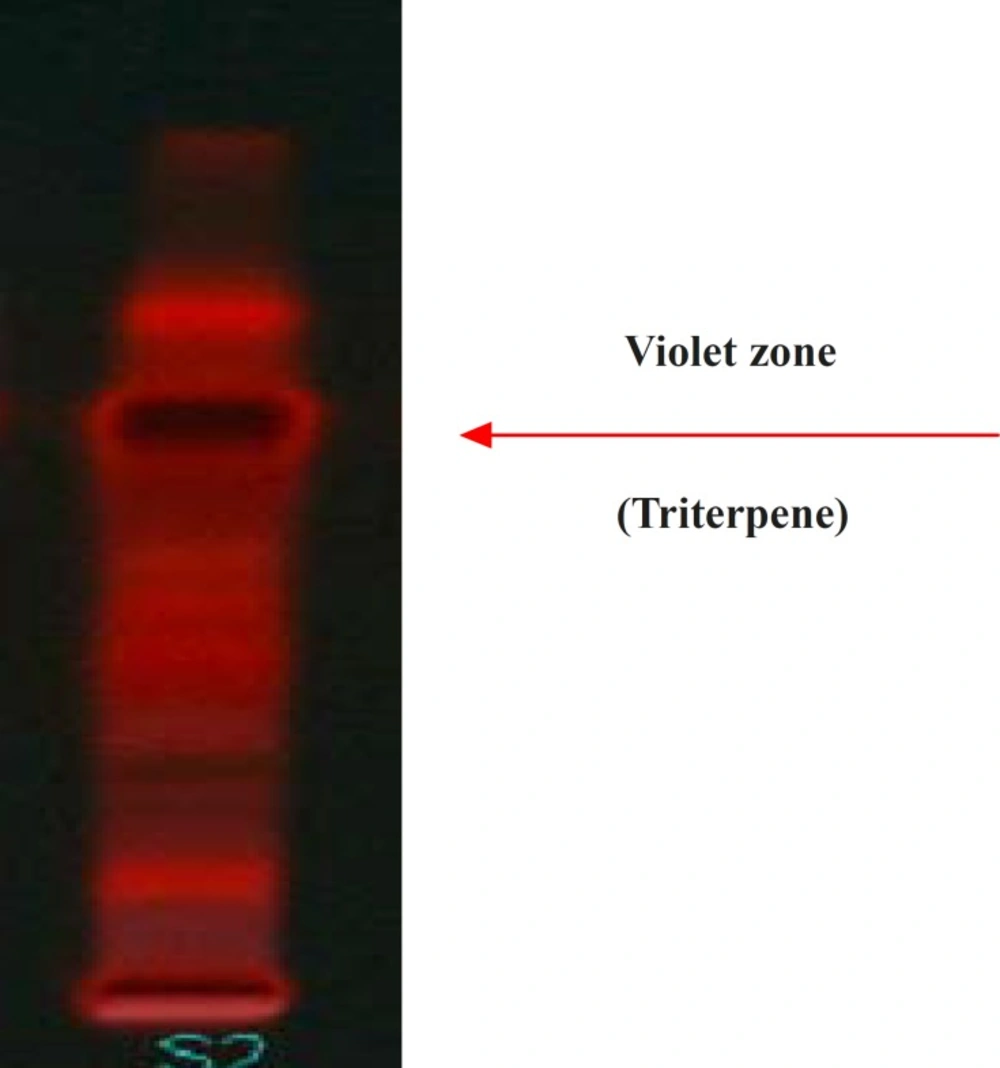
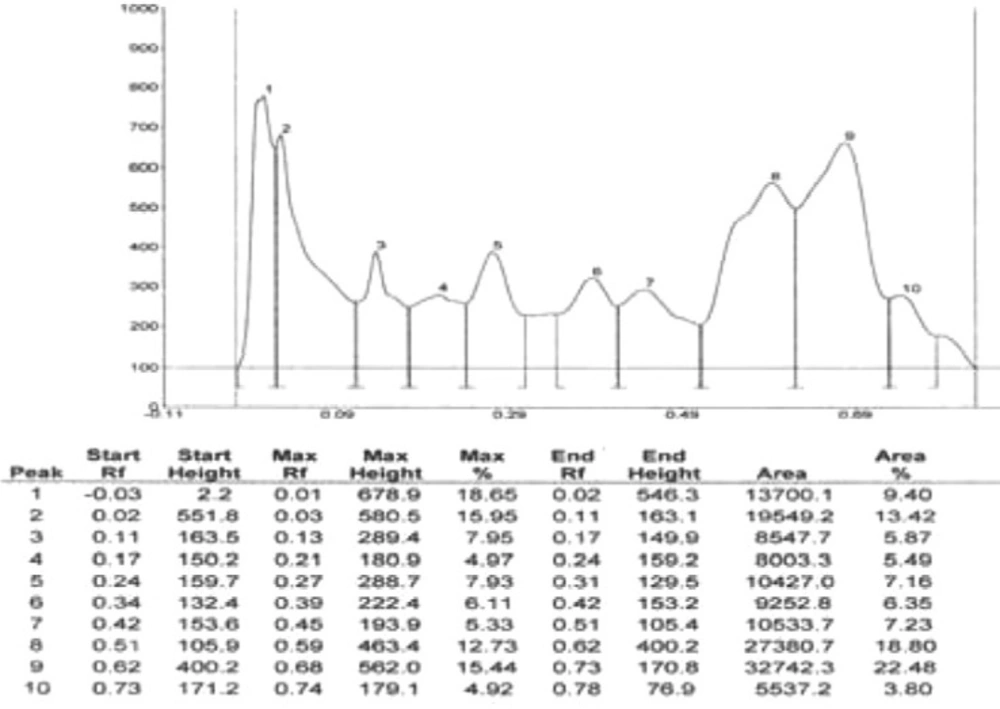
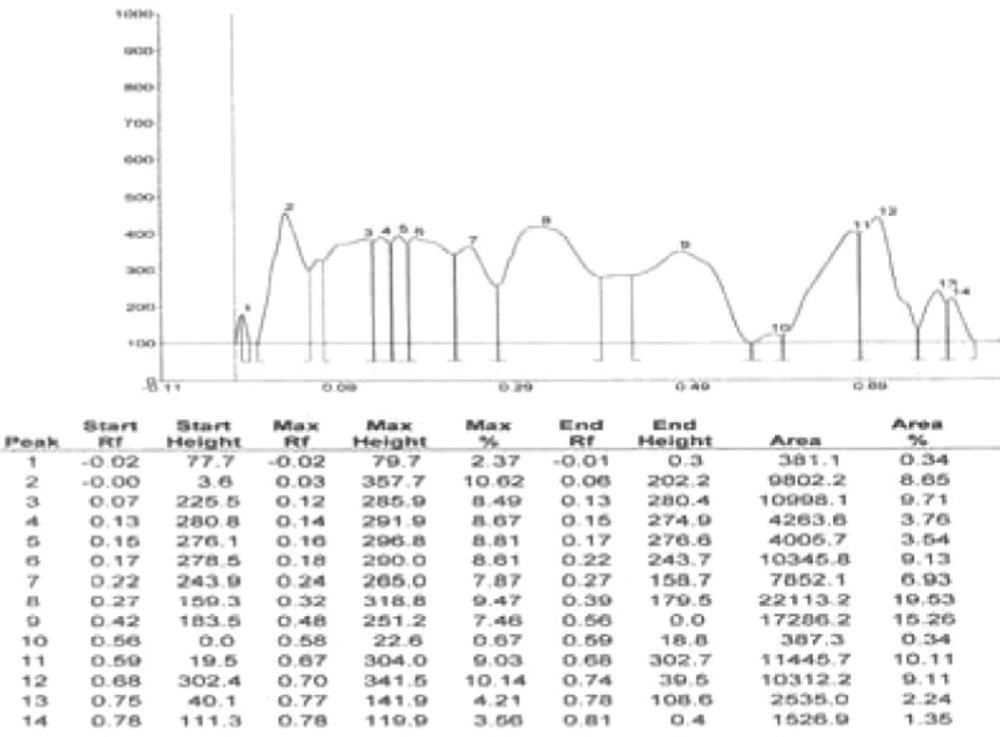
Results of HPTLC studies indicated that the ethyl acetate extracts of Pogostemon parviflorus leaves (17) contain triterpene, showing 10 uv-absorption peaks in 254 nm, 14 peaks in 366 nm and a violet zone in visible wavelengths after derivatization with anisaldehyde sulphuric acid (Figures 5-9). This compound may account for the anti-dermatophytic activity of this plant. This finding was in agreement with a previous study reporting that the ethanolic extract of Pogostemon parviflorus leaf possesses antifungal properties against dermatophytic species isolates (18).
Briefly, based on the results of this study, we can consider the ethanolic extract of Pogostemon parviflorus as a new source for developing local antifungal agents. However, further studies are needed to determine the efficacy of active chemical constituents of this plant extract. Toxicological studies must also be performed to ensure the safety of the extract.