Introduction
The most common cause of food and water-borne E. coli-mediated human diarrhea is enterotoxigenic Escherichia coli (ETEC), worldwide. In the developing countries, the incidence of human enteric diseases caused by ETEC infections is approximately 650 million cases per year, resulting in 800,000 deaths, primarily in children of below five years old (1, 2). Therefore, an effective vaccine against ETEC for using in children living in ETEC-endemic countries and in travelers is highly required (3).
The heat-labile enterotoxins of E. coli (LT) is one of the major virulence determinants for ETEC. It consists of a toxic A subunit (LTA) and a pentamer of receptor-binding B subunit (LTB) (4). The LTB bind to the gangliosides on the host cell and the A subunit enters the cell, leading to an increase in cAMP and consequently diarrhea (2). The immune-modulatory and immunogenicity properties of LTB have been extensively studied and led to its usage as a powerful candidate as immunogen and adjuvant (5, 6) in vaccine development (7).
Many vaccine candidates consisting of toxin-derived antigens either purified or expressed on the bacterial surface as live-attenuated ETEC vaccines (8, 9) have been studied and are in different stages of testing (3). Live attenuated bacteria can be engineered to deliver foreign antigens to the immune system. They are attractive vaccine vectors as they have the possibility of protection at mucosal surfaces (6, 10 and 11). The convenience to inoculate, well suited large-scale manufacturing, potential stability without refrigeration (via lyophilization) (12), strong cellular and humoral immunity induction, and also potential to be immunologic adjuvant are the other advantages of the live bacterial vaccines (10, 13).
Growing number of reports published over the last two decades have indicated the effectiveness of avirulent Salmonella strains as a delivery system for recombinant heterologous antigens to stimulate protective immune response against different infectious diseases in animal models (14, 15). Several attenuations have been constructed in Salmonella strain to present it as avirulent while preserving different degrees of invasiveness and thus immunogenicity (16). Our group have been interested in developing a novel ETEC vaccine based on recombinant Salmonella strains PhoPc, which was attenuated for survival within macrophage (17).
Expression of a specific foreign antigen may impose considerable metabolic stress on the attenuated live vector strain, which may consequently limit its in-vivo immunogenicity. Furthermore, the in-vivo instability of foreign antigen which may be caused from unregulated and high levels of foreign protein expression is one potential drawback of using live bacteria vaccines such as S. typhimurium for antigen delivery. This problem can be circumvented by using in-vivo-inducible promoters which stabilized expression of heterologous antigens in bacterial vaccine vectors (18). The level of foreign antigen expression is low under the control of this promoter until the vector bacterium receives an environmental stimulus in-vivo. Consequently, they stabilized the expression of foreign antigen in live condition (18). Totally, the proper promoter to control the expression of the foreign antigen should be carefully selected for certain antigen (19).
Herein, the effect of promoter type on the immune responses elicited by the Salmonella-based live vaccines expressing LTB was examined. The in-vitro and in-vivo activities of constitutive tac promoter, IPTG inducible trc promoter which have the ability to drive high-level expression of certain antigens (20) and in-vivo-induced promoters were compared (20). In this study the use of the in-vivo-induced nirB promoter and further derivative, which was previously constructed by our group to remove the ability of chemical induction from nirB promoter (21), were investigated for expression of LTB in attenuated Salmonella live vector vaccine. The nirB promoter directs the expression of an operon that includes the nitrate reductase gene. This in-vivo-inducible promoter is regulated by nitrate as well as nitrite and becomes active under anaerobic conditions e.g. inside eukaryotic cells including the macrophages (22, 23). Entry of salmonella into cells activated this promoter (23). In-vivo studies showed that the nirB promoter can be a highly efficient expression system for live vaccine delivery (24). Many studies demonstrated Salmonella strains expressing different antigens from nirB promoter induce higher immunity than the same strains expressing similar antigens under the control of a constitutive promoter (22, 23).
Many studies administered Salmonella-vectored vaccines via nasal route in mice (25, 26) and demonstrated superior immunogenicity compared to the oral administration (27). Now, the Salmonella-vectored vaccines engineered to express LTB were examined in mice after nasal immunizations for stimulation of serum anti-LTB IgG antibody responses. Herein, the influence of in-vitro promoter activity on the induced antibody response elicited in the mice nasally vaccinated with recombinant Salmonella strains was evaluated.
Experimental
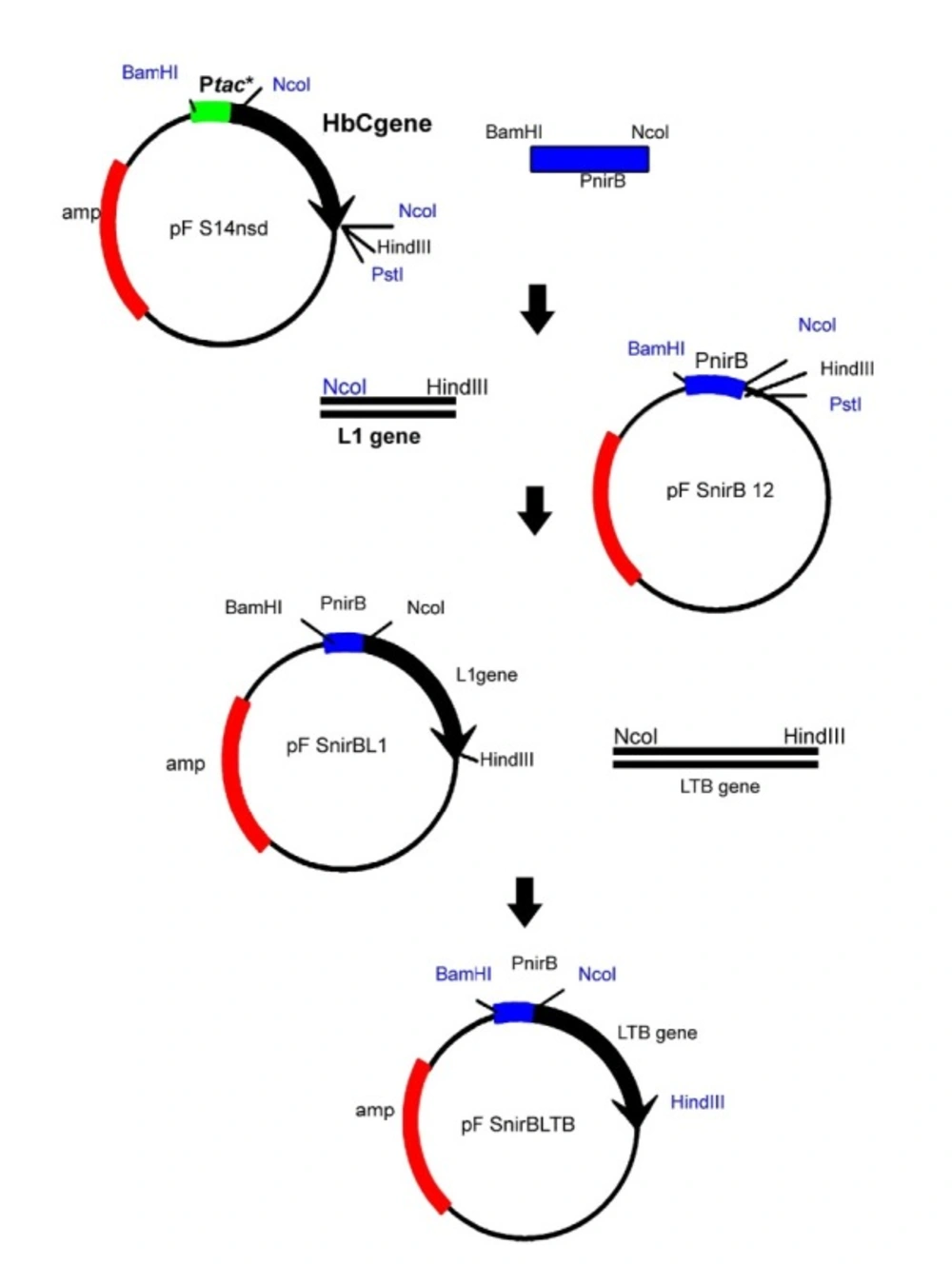
Plasmids construction
To amplify nirB promoter, the forward primer (nirBam): (5′CGTTGGATCCAGCTGTCCGCAGGCG3′) including BamHI restriction site (underlined) and reverse primer (nirNco): (5′ CTGACTGCAGCCATGGTTGCCTCGATTTC 3′) including NcoI restriction site (underlined) were designed based on the nitrite reductase sequence (X14202 gi:42120) and the PCR reaction was carried out using DH5α a E. coli genome as template. The LTB gene was ampliefied as previously reported (28). Briefly, the designed primers based on LTB gene (Gene bank accession number: J01646) were used to amlify LTB gene from a plasmid DNA of clinical isolate of enterotoxigenic containing LT operon as template. As demonstrated in Figure 1, to construct pnirBLTB, firstly the amplified nirB BamHI-NcoI fragment was cloned in pFS14nsd which is an expression vector containing HBcAg under the control of tac promoter with a synthetic Shine-Dalgarno sequence 8 bases from the HBcAg AUG (29), a kind gift from Florian Schödel, Centre Hospitalier Universitaire Vaudois (CHUVD), Lausanne, Switzerland. The resulting plasmid designated pnirB12, was used to construct pnirBL1 by inserting the NcoI-HindIII fragment encoding the HPV16-L1 open reading frame (17). Then, the L1 fragment was exchanged by the amplified LTB gene (21) containing NcoI and HindIII on its end sides.
pnirB78-23LTB was constructed by two-step cloning as explained in our previous study (21). Briefly, the synthetic nirB78-23 promoter was cloned in a pkk223 derivative plasmid and then the amplified LTB gene was cloned downstream of the nirB78-23 promoter in NcoI-HindIII sites.
For construction of ptacLTB, the LTB coding sequence flanking with NcoI in 5′ and HindIII in 3′ (21) was inserted in the place of L1 coding sequence in plasmid pFS14nsd HPV16-L1S (30), a kind gift from Florian Schödel, CHUVD, Lausanne, Switzerland. The construction of ptrcLTB expressing the LTB under the control of the trc promoter was described earlier (28). Briefly, the LT-B gene was cloned from pUCLTB into pTrc 99.
All the resulting plasmids were introduced by electroporation into the attenuated S. typhimurium strains PhoPc (31), attenuated in both virulence and survival within macrophages by introduction of a point mutation in the phoQ gene (16).
Salmonella Competent cells preparation and transformation
To prepare Salmonella competent cells, the overnight cultures of different clones were diluted 1:100 and used to inoculate fresh broth LB and incubated at 37 °C with shaking to an optical density (OD)600 of 0.75. Next, the cells were chilled in an ice-water bath for 15 min and then were harvested by centrifugation (4,000×g, 10 min, 4 °C). Then, the cells were washed twice with ice-cold 15% glycerol. Finally, they were suspended in ice cold 15% glycerol. The plasmids were transformed into S. typhimurium strains PhoPc by electrotransformation with a gene pulser (Biorad) set at 1.8 kV, 25 μF, and 200 Ω. After electroporation, the cells were transferred to a sterile culture tube containing SOC media (2% w/v tryptone, 0.5% w/v yeast extract, 8.56 mM NaCl, 2.5 mM KCl, 10 mM MgCl2 (anhydrous), 10 mM MgSO4 (heptahydrate) and 20 mM glucose) and incubated at 37 °C, with shaking at 150 rpm, for 1 h to allow expression of the antibiotic resistance gene. Then, the transformation mixture was centrifuged and the cells were plated on LB plates containing ampicillin (50 μg/mL) and 5-Bromo-4-chloro-3-indolyl-phosphate toluidine salt (BCIP) (50 μg/mL).
Quantitation of expression level
To compare the expression level of rLTB from recombinant PhoPc under nir B78-23, trc and tac promoters, ng rLTB/109 colony-forming unit (cfu) of each strain was calculated. Accordingly, PhoPc containing pnirBLTB, pnirB78-23LTB, ptrcLTB, and ptacLTB were grown in LB medium containing ampicillin (100 μg/mL) to an OD600 of 0.4-0.6. The PhoPc/pnirBLTB and PhoPc/pnirB78-23LTB were induced by sodium nitrite (2.5 mM), and sodium nitrate (20 mM) under both aerobic and anaerobic conditions as described previously (21). In addition, a reaction without chemical induction was also carried out. In case of PhoPc/ptrcLTB, and PhoPc/ptacLTB, the induction was performed by adding IPTG (0.4 mM). All of the recombinant PhoPcs was incubated for 4 h after induction. Then, cfu of each sample culture was determined. Briefly, the serial dilutions of the samples were made and 0.1 mL of each bacterial concentration was plated on ampicillin containing LB agar plate. After overnight incubation at 37 °C, the numbers of colonies on the plate were counted and then the cfu/mL vs. OD600 were plotted.
In the next step, the cells were harvested and then lysed by freezing and thawing three times with vigorous shaking. Afterwards, rLTB was measured in the crude lysates and supernatant using GM1-ELISA. The recombinant LTB concentration was calculated as ng/109 cfu.
GM1-ELISA to evaluate rLTB expression
The amount of rLTB levels was measured by the GM1-ELISA method as describe previously (21). Briefly, polystyrene 96-well microtiter plate was coated with 5 μg ganglioside GM1 type III (Sigma) per well diluted in carbonate buffer (pH 9.6) and incubated 6 h at room temperature. Further, the plate was incubated with PBS-BSA (0.1% (w/v) (Bovine serum albumin (BSA) in phosphate-buffered saline (PBS)) for 30 min at 37 °C. Then, after washing the wells with PBS-T (0.05% (v/v) Tween-20 in PBS), 100 μL of PBS-BSA containing serially diluted standard LT (10 μg/mL) (Sigma) as positive control, diluted supernatant, and bacterial lysate of samples (1:10 to 1:10000 in PBS) were added and incubated for 1 h at 37 °C. After another washing procedure with PBS-T, the wells were incubated with 100 μL anti-LTB/cholera toxin B subunit cross reactive monoclonal antibody D15-8 (kindly provided by the Pasteur Institute, Paris) and LT39 monoclonal antibody (kindly provided by Svennerholm, university of Goteborg, Sweden) (1:10000) as the first antibody for 1 h at 37 °C. After a final washing procedure, 100 μL of tetramethyl benzidine (TMB) (Abcam) and H2O2 (at a final concentration of 0.03%) were added as the substrate, followed by incubation for 15 min at 37 °C. Then, the reaction was interrupted with 2N H2SO4. OD was measured with a spectrophotometer (Awareness Technology, Inc.) at a wavelength of 450 nm.
To evaluate the amount of rLTB expressed using different promoters in PhoPc, a standard curve of reference LT was generated and the amount of rLTB in each sample was determined by interpolation on standard curves. The production rate of recombinant LTB was reported as ng/109 cfu.
Immunization and serum collection
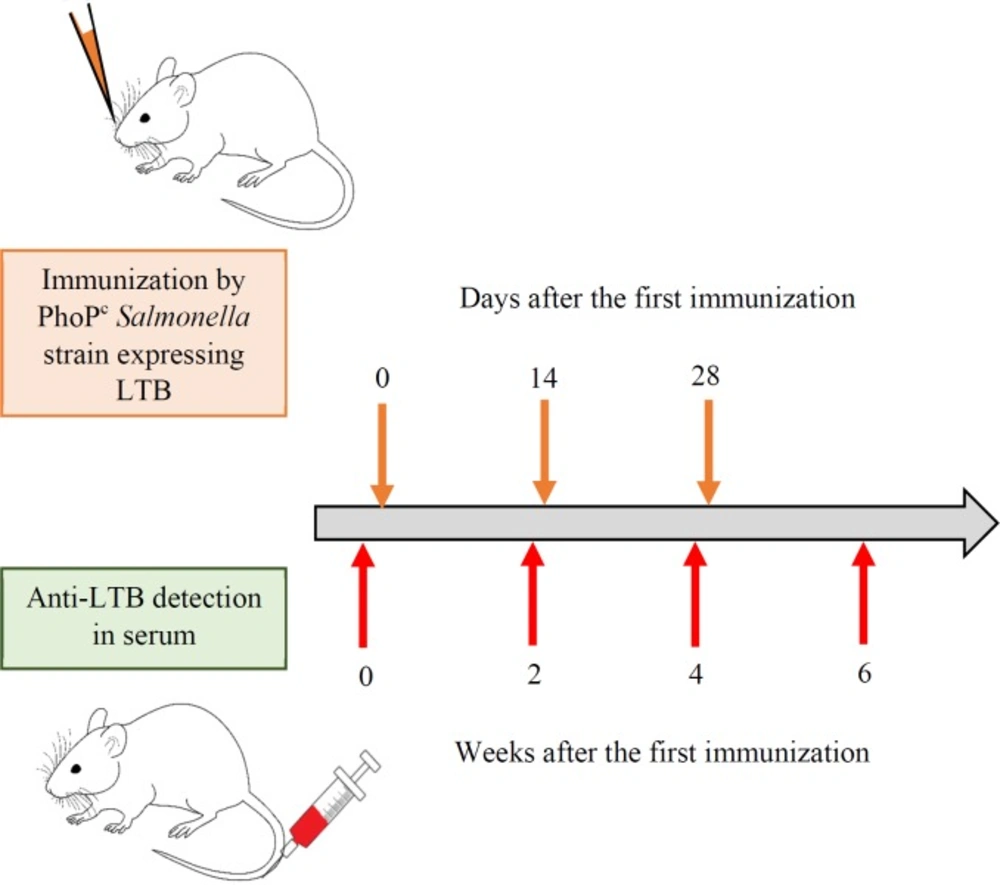
PhoPc/pnirBLTB, PhoPc/ptrcLTB, PhoPc/ptacLTB, PhoPc/pnirB78-23LTB and PBS were used for nasal immunization. Accordingly, a single colony of each PhoPc was grown at 37 °C in LB broth containing 100 μg/mL ampicillin to an OD600 of 0.6 to 0.8. Following centrifugation at 5,000´g for 10 min, the bacterial pellet was resuspended in 1 mL PBS to yield 0.5-5 1011 cfu/mL. The bacterial suspension was diluted in PBS to reach 109 cfu per 20 mL.
Four- to eight- weeks old BALB/c mice (Semnan University of Medical Sciences) were anesthetized and 20 m L of bacterial suspension containing 109 cfu of each recombinant PhoPc strain was administrated by intranasal application via a Pasteur pipette. The booster doses were administrated on day 14 and 28. The blood samples were collected from the tail vein of the mice before the first immunization (0) and also 2, 4 and 6 weeks after the first dose administration (Figure 2). The serum was extracted from the whole blood by centrifugation at 4,000g for 5 min and stored at -20 °C.
Detection of antigen-specific serum antibody responses
The humoral immune responses to the LTB antigens were measured in the blood samples taken before (0) the first dose administration as well as 2, 4 and 6 weeks after the first immunization. ELISA plates were coated with 5 μg ganglioside GM1 and incubated at room temperature for 6 h. Then, the wells were blocked with PBS-BSA at 37 °C C for 30 min. In the next step, 100 mL of the purified LT toxin (10 μg/mL) was added to the plates and incubated for 1 h. Next, after 3 times rinsing with PBS-T, the plates were incubated for 1 h with 100 μL of serially diluted various mouse sera in PBS buffer. After a second washing step, the bound antibodies were detected using HRP conjugated goat anti-mouse antibodies at a dilution of 1:10000 in blocking solution. Immune complexes were developed with TMB in the presence of 0.03% H2O2 and the reactions were stopped after addition of 2M H2SO4.
Statistical analysis
Graph-Pad Prism 6.0 for Windows (Graph-Pad Prism, San Diego, California, USA) was used to perform Statistical analysis. To study in-vitro rLTB expression and in-vivo anti-LTB antibody responses, one-way ANOVA with Tukey’s post-hoc test was applied in the current study. The data were presented as mean ± standard deviation (SD). The p-value less than 0.05 (p < 0.05) was considered statistically significant.
Results
Expression of rLTB from PhoPc harboring nirB and synthetic nirB promoters
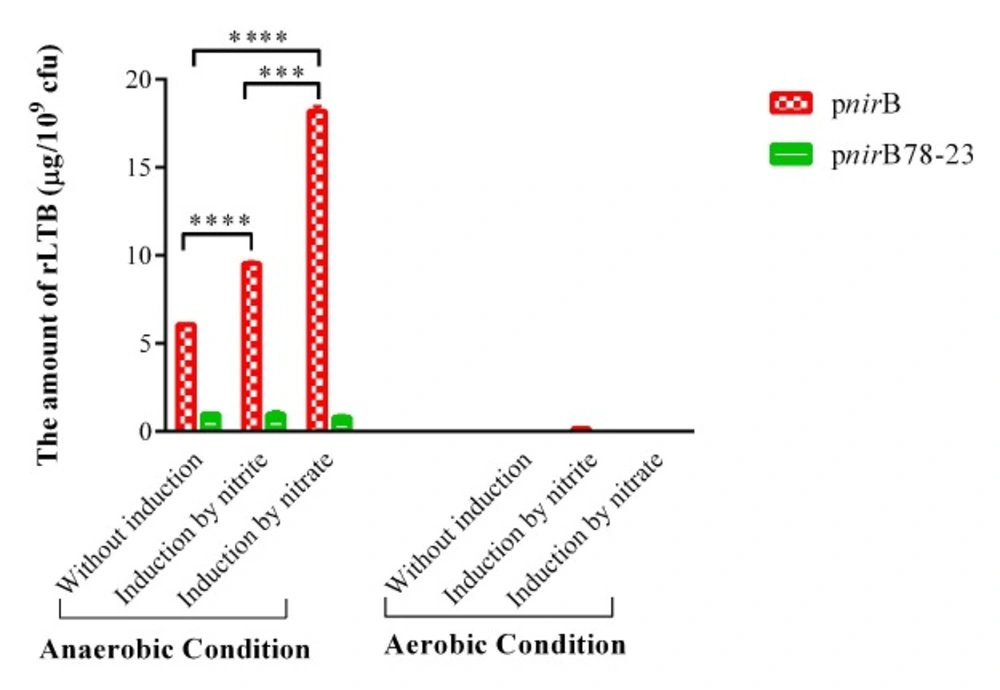
PhoPc containing LTB-expressing plasmids under the control of nirB and synthetic nirB promoters were grown under a number of in-vitro conditions, and the supernatants were analyzed for rLTB expression by the GM1-ELISA method. No LTB was expressed in negative control, PhoPc containing plasmids with nirB and synthetic nirB promoters, and without LTB gene (data were not shown). PhoPc produced significant higher amount of rLTB in anaerobic compared to aerobic condition (Figure 3). As demonstrated in Figure 3, in anaerobic condition, both nitrate and nitrite significantly increased nirB promoter activity in PhoPcSalmonella strain in comparison with uninduced condition (pnirB nitrate and nitrite induction vs. uninduced condition (p < 0.0001)). The nirB promoter activity was enhanced 1.6- and 3-fold over uninduced PhoPc/pnirBLTB in response to nitrite and nitrate, respectively. Significantly higher amount of rLTB was obtained in PhoPcSalmonella by nitrate comparing to nitrite induction (pnirB, nitrate vs. nitrite induction, p < 0.0001). The efficacy of nitrate to induce nirB promoter in PhoPcSalmonella under anaerobic condition was 1.9-fold of that of nitrite. However, rLTB expression under nirB promoter in aerobic condition was not enough to be detected by ELISA technique. Nitrite partly induced nirB promoter in PhoPcSalmonella strain under aerobic condition.
PhoPc with synthetics nirB promoter (21) did not produce detectable amounts of rLTB when grown in aerobic condition even in the presence of nitrate or nitrite as inducers. Under anaerobic condition, the activity of synthetics nirB promoter was 16% of native promoter. When PhoPc containing synthetics nirB promoter was grown under anaerobic condition, neither nitrite nor nitrate could induce rLTB expression.
In-vitro analysis of rLTB expression by PhoPc uder tac and trc promoter
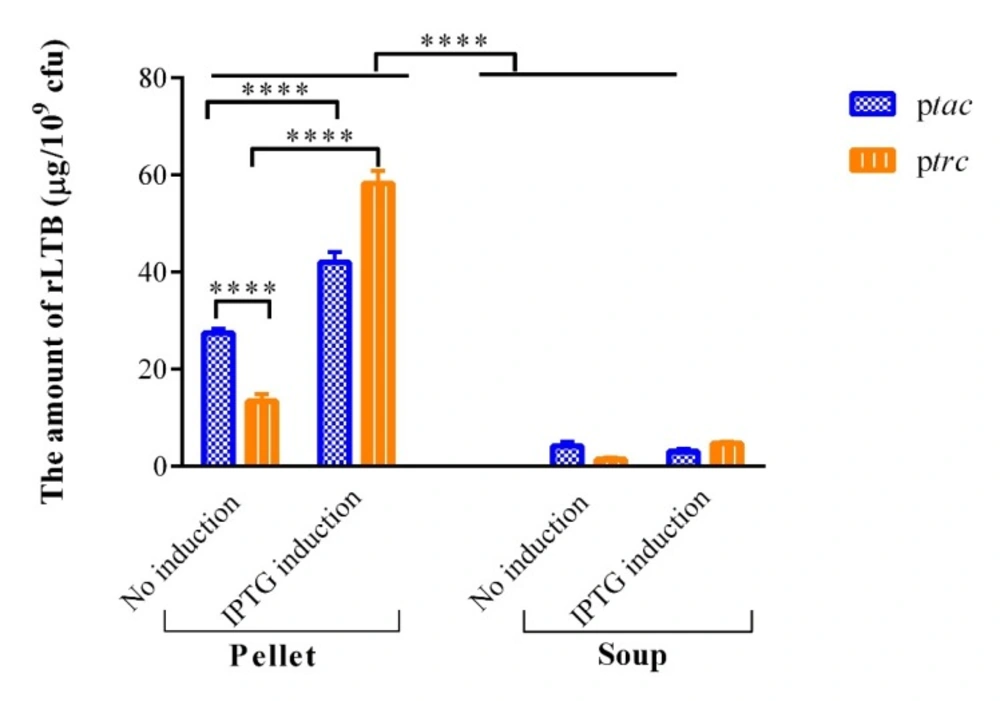
The expression of recombinant LTB in the lysates and supernatant of PhoPc harboring LTB-coding plasmids with tac and trc promoters was compared by the GM1-ELISA method. The rLTB was not detected in PhoPc stain harboring plasmids with tac and trc promoters and without LTB gene (data were not shown). As indicated in Figure 4, the rTLB constitutive expression (without IPTG induction) under tac promoter was significantly lower than the induced expression in PhoPc (tac induced (pellet) vs. uninduced (pellet), p < 0.0001).
The results of this study demonstrated that trc promoter is active in both induced and uninduced conditions. IPTG induction led to significantly more rLTB expression (4.3-fold) in PhoPc harboring trc promoter compared to uninduced condition (trc induced (pellet) vs. uninduced (pellet), p < 0.0001). However, the tac promoter in uninduced condition resulted in significantly higher rLTB expression as compared to trc promoter in the same condition (Figure 4, tacvs. trc uninduced condition, p < 0.0001).
Furthermore, rTLB was secreted to supernatant in both uninduced and induced conditions under tac and trc promoters in PhoPc. However, the amount of secreted rTLB is significantly less than in bacterial pellet in both uninduced and induced expression (pellet vs. soup in both induced and uninduced conditions under both trc and tac promotes vs. the corresponding conditions, p < 0.0001). Totally, it can be concluded that PhoPc expressing rLTB under tac and trc promoter has the ability to be used as live vaccine vector.
Anti-LTB humoral responses after nasal immunization
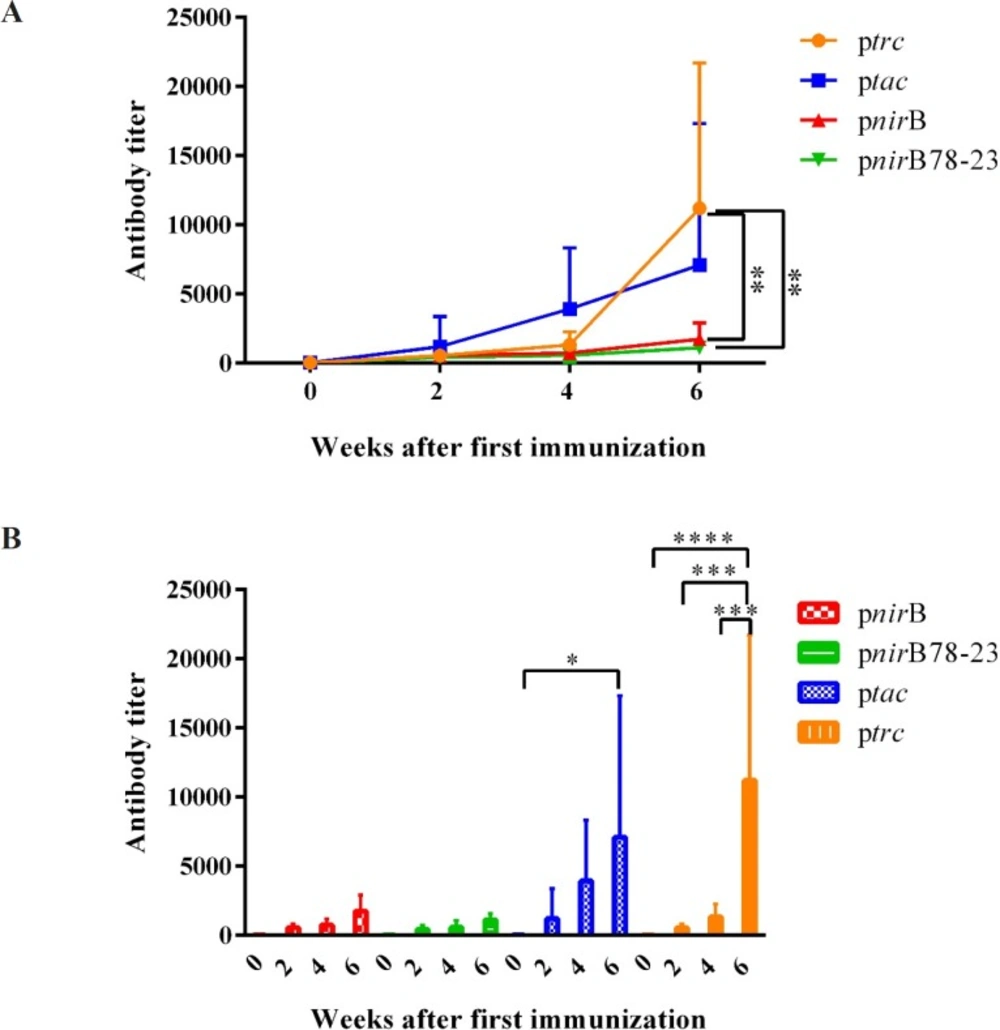
BALB/c mice were immunized intranasally with PhoPc/pnirBLTB, PhoPc/pnirB78-23LTB, PhoPc/ptacLTB, PhoPc/ptrcLTB, and PBS. The sera were analyzed for the presence of anti-LTB antibody by an ELISA method just before (0), 2, 4 and 6 weeks after the first immunization. No anti-LTB antibody was detected in PBS-vaccinated mice (data were not shown). In response to the first dose of vaccination, the highest antibody response was elicited by PhoPc/ptacLTB. However, no significant differences among various groups were demonstrated after first vaccine dose. The second vaccination dose (the first boosting dose) led to more than 3-fold increase in antibody titer of the mice vaccinated with PhoPc/ptacLTB. However, there were no significant differences between various groups at this time.
The second boosting dose caused approximatey 20-fold increase in Anti-LTB antibodies in the mice received PhoPc/ptrcLTB. The serum anti-LTB titers induced by the second boosting dose were increased 6-, 3.2- and 2.6-fold in the mice immunized by PhoPc/ptacLTB, PhoPc/pnirBLTB and PhoPc/pnirB78-23LTB, respectively. As demonstrated in Figure 5A, the mice receiving PhoPc strains expressing LTB under the control of the trc promoter demonstrated significantly higher anti-LTB antibody compared to the mice receiving PhoPc strains expressing LTB under the control of the nirB78-23 and nirB promoters, 2 weeks after the second boosting dose (p < 0.01). Totally, the data obtained in the mice using nasal immunization suggested that the PhoPc strain expressing LTB from the constitutively active trc promoter is the superior immunogenic one.
Although PhoPc/pnirBLTB and PhoPc/pnirB78-23LTB were able to induce anti-LTB specific antibody by single dose, there was no significant increase in antibody titer even after the second boosting dose. As demonstrated in Figure 5B, the second boosting dose in PhoPc/ptacLTB caused significant enhancement in serum anti-LTB titer (tac 0 vs. 6 weeks after the first immunization, p < 0.05). In addition, each boosting dose of PhoPc/ptrcLTB led to a significant increase in anti-LTB titers of the vaccinated mice (trc 0 vs. 6 weeks after the first immunization, p < 0.0001; 2 vs. 4 and 4 vs. 6 weeks after the first immunization, p < 0. 001).
Discussion
In the present study, the development of a novel ETEC vaccine based on recombinant Salmonella strains expressing LTB was reported. Herein, the strong constitutive tac and IPTG-inducible trc promoters as well as the in-vivo-inducible nirB and synthetic nirB were evaluated in Salmonella to quantify in-vitro antigen levels and to simultaneously monitor in-vivo antibody response. In previous studies, a range of different antigens have been expressed under the control of tac, trc and nirB promoters, in Salmonella strain and some of them have induced protective immunity (17, 32-35).
Herein, in-vitro studies demonstrated that PhoPc can produce rLTB with critical characteristics such as pentameric formation, binding to its receptor (ganglioside GM1), and conservation of immunogenic epitops under the control of both constitutive and in-vivo inducible promoters. In addition, the uninduced expression of rLTB in PhoPc strain under tac and trc promoter was confirmed. High basal level of transcription and the leakage of trc promoter which cause protein expression in uninduced cultures have been proved in previous studies (36, 37).
We previously constructed a synthetic nirB promoter in which the responding regions for nitrite and nitrate inducers were not considered. Consequently, the expression of recombinant protein is induced only under low oxygen pressure or anaerobic conditions (21). The synthetic promoter demonstrated less activity than native nirB promoter in anaerobic condition in PhoPc Salmonella strain. In agreement with our previous report in E. coli (21), the synthetic nirB promoter did not respond to chemical inducers (nitrite and nitrate) of intact native nirB promoter in PhoPcSalmonella strain. It was previously reported that the nature of the protein expressed under nirB and synthetic nirB may adversely affect due to anaerobic growth of bacteria (21). Herein, the pentameric formation of the expressed LTB which is necessary for binding to its receptor, immunogenicity and adjuvanticity was confirmed by interaction with LT39 antibody that is specific for the pentameric structure of LTB not for the monomer form (38). In addition, Guidry et al. demonstrated that the toxicity, immunogenicity, and oral adjuvanticity of LT are dependent on binding of the B subunit to ganglioside GM1 (39). Therefore, the results of GM1-ELISA demonstrated that receptor recognition and immunogenic determinants of the expressed LTB directed by different promoters are conserved.
Construction of pnirBLTB. Firstly, a plasmid designated pnirB12 was constructed by cloning of the amplified nirB BamHI- NcoI fragment in pFS14nsd, an expression vector containing HBcAg under the control of tac. Then, the NcoI-HindIII fragment encoding the HPV16-L1 open reading frame was inserted to pnirB12. Finally, the L1 fragment was replaced by the amplified LTB gene containing NcoI and HindIII on its end sides
Schematic representation of immunization and anti-LTB detection. The bacterial suspension (109 cfu) of different recombinant PhoPc strains was administrated nasally after anesthetization. The booster doses were administrated two and four weeks later. The blood samples were collected form the tail vein of the mice the day before (0) of first immunization and also 2, 4 and 6 weeks after the last booster dose
rLTB expression from PhoPc containing nirB and synthetic nirB promoters. rLTB expression was analyzed in the supernatants of PhoPc containing expression plasmids of LTB under the control of nirB and synthetic nirB promoters by the GM1-ELISA method. The data represent mean ± SD of triplicate wells. *** and **** represent for p < 0.001 and p < 0.0001, respectively
In-vitro analysis of rLTB expression from PhoPc under the control of tac and trc promoters. The comparison of rLTB in the lysates and supernatant of PhoPc harboring LTB-coding plasmids with tac and trc promoters was carried out by the GM1-ELISA method. Values are mean ± SD of triplicate wells. ****represents for p < 0.0001
LTB specific IgG in serum after nasal immunization with PhoPc harboring different promoters. Different groups of BALB/c mice were nasally immunized with 109 cfu of PhoPc harboring nirB, trc, tac, and nirB78-23 promoters. Anti-LTB titer in the blood samples of the mice was determined the day before the first immunization (0), and also 2, 4 and 6 weeks after the first immunization. Data are expressed as the means ± SD of antibody titer of the mice in each group. (A) represents the significant differences of different groups 6 weeks after the first immunization dose. (B) demonstrates increase in antibody titer in each group after booster doses. *, **, *** and **** represent for p < 0.05, p < 0.01, p < 0.001, and p < 0.0001, respectively
Nasopharynx-associated lymphoid tissue (NALT) appears to play a key role in induction of immune response in rodents. NALT involves M, antigen presenting cells (APCs), T and B cells and consequently provides the requirements for a protective immune response induction (25). Many studies reported higher antibody titers via the intranasal route compared to oral inoculation of mucosal vaccine (10, 25, 40 and 41). Several studies administered Salmonella-vectored vaccines via nasal route in mice (26, 42 and 43). Galen et al. demonstrated superior immunogenicity of intranasal Salmonella typhi live vector rather than its oral administration. They explained that the poor performance of orogastric immunization is due to the high exposure of gut to bacteria, other microorganisms and food antigens that may suppress immune responses and limit the magnitude and duration of the induced immunity (27). Therefore, after successful in-vitro expressing of LTB in Salmonellatyphimurium PhoPc vaccine strain under the control of different promoters, we aimed to determine whether the expressed vaccine antigen could elicit humoral immune response in nasally vaccinated animals.
In the present study, we demonstrated that significant higher anti-LTB antibody titer in response to second boosting dose was achieved when the mice were vaccinated by PhoPc/ptrcLTB comparing to PhoPc/pnirB78-23LTB and PhoPc/pnirBLTB vaccinated mice. In agreement with our results, Dunstan et al. demonstrated that the native nirB promoter was not able to direct expression of immunogenic levels of C fragment in ΔaroAD mutant of Salmonella. In order to circumvent the inability of the nirB promoter for directing expression of immunogenic levels of C fragment, they incorporated optimal RBS sequence containing complementary sequence of E. coli 16S rRNA and also optimal size of the spacer region between the RBS and ATG, into the nirB promoter construct. They demonstrated that the incorporation of the optimal RBS had no effect on the anti-tetanus toxoid antibody generated in the mice immunized with S. typhimurium strains expressing the C fragment of tetanus toxin using nirB promoter. Therefore, they concluded that the poor translation initiation is not responsible for the lack of immune response from the nirB promoter cassette (44). However, some studies reported that Salmonella strains expressing antigens under nirB promoter demonstrate superior immunogenicity than the similar strains in which the expression of the same antigens is controlled by a constitutive promoter (23, 35). For example, our results are in contrast with the study of McSorley et al. in which gp63 expressing under either inducible (nirB and osmC) or constitutive (tac) promoter in S. typhimurium were compared. They demonstrated that nirB shows superior plasmid stability which correlated with the increased immunogenicity to the heterologous antigen gp63. Furthermore, significant cellular immune responses were detected only in the mice immunized with a single dose of S. typhimurium expressing gp63 under nirB promoters. However, these responses were not detected in the mice vaccinated with a single dose of S. typhimurium expressing gp63 under either tac or osmC (18).
The aim of the most studies using attenuated strains of Salmonella as live vaccine vectors is to determine the most important factor for the development of an immune response against a foreign antigen (45). In earlier study, it was demonstrated that the immunogenicity of the vaccine is influenced by the amount of antigen produced and consequently the choice of promoter. It was believed that more antigen production by strong constitutive promoters lead to more immunogenic vaccine (10). Cardenas et al. demonstrated that the initial amount of antigen that primes the GALT is more important than the persistence of the vector in tissues for eliciting immunity against a foreign antigen orally administrated by attenuated strains of Salmonella (46). However, some studies demonstrated that in-vitro study of antigen expression by live vaccine may not accurately correlate to in-vivo immunity. For example, John et al. compared the strong constitutive tac promoter, the in-vivo induced htpG promoter, and the in-vivo-induced iron-regulated irgA promoter for the expression of B subunit of cholera toxin (CtxB) in V. cholera. They demonstrated that under in-vitro condition, the expression was greatest and equivalent from the tac promoter and the irgA promoter when under low-iron conditions. Although the gut is a low-iron environment, V. cholerae strains expressing CtxB from the tac promoter directed superior immune responses in animals (20). In another study, despite the fact that the levels of in-vitro expression of C-fragment of tetanus toxin under in-vivo-inducible pagC promoter and constitutive trc promoter in Salmonella appeared to be equivalent, it was found that the pagC promoter stimulates greater immune responses than the trc promoter (44). In a study by Chatfield et al., in-vitro study indicated equivalent or higher expression levels of C-terminal of tetanus toxin (Frg C) in Salmonella strain under the control of tac promoter in comparison with the strain with the pnirB-Frg C construct. However, less immunity against tetanus was achieved in the mice immunized with Ptac-Frg C Salmonella strain (35).
In our study, in-vitro studies demonstrated that more rLTB is expressed by the constitutive tac promoter compared to the uninduced trc promoter in bacterial soup (4.2 ± 0.92 vs. 1.4 ± 0.46 μg/109 cfu( and also pellet (27.4 ± 0.89 vs. 13.4 ± 1.42 μg/109 cfu, p < 0.0001). Furthermore, nirB promoter directed more LTB expression in PhoPc/pnirBLTB under anaerobic condition without induction compared to the amount of rLTB secreted by PhoPc/ptrcLTB in bacterial soup (6.06 ± 0.05 vs. 1.4 ± 0.46 μg/109 cfu, p < 0.01). However PhoPc strains expressing LTB under the control of the trc promoter stimulated superior immunity. Our results indicated that of the promoters examined, the trc promoter may be the best suited promoter for expression of LTB in PhoPc strains. Totally, consistent with the results of Dunstan et al. it can be concluded that the strain’s immunogenicity poorly correlates with the total amount of heterologous antigen that a vaccine strain produces (44). Due to the complexity of living vectors interactions with mammalian hosts, in-vitro study is not enough to predict in-vivo responses (10). A number of other variables, such as the location, timing, as well as the identity of the antigen to be expressed and the type of required immune response may have influence on the efficacy of vaccine based on attenuated Salmonella (20, 44).
In summary, anti-LTB humoral response was used as a surrogate marker to compare in-vivo activity of various promoters in PhoPc. Despite the fact that the level of rLTB expressed constitutively in-vitro (without IPTG) under tac promoter was more than that expressed under trc promoter, it was found that immunization using recombinant PhoPc under the control of trc promoter results in the superior humoral immunity. Consistent with Dunstan et al., we concluded that the level of in-vitro antigen expression is not the only factor affecting the efficacy of attenuated Salmonella-based vaccine (44).