Introduction
Costs on new drug discovery researches have compelled medicinal chemists to look for new strategies to obtain novel active pharmaceutical ingredient (1). One of successful and low-cost strategies is the clustering of the established therapeutic drug agents on a suitable molecular scaffold in order to improve their pharmaceutical efficiencies (2).
The clinical term myotonia (muscle tension) is a skeletal muscle stiffness and it is characterized by abnormal delayed relaxation of the skeletal muscles after electrical stimulation or contraction (3). Mexiletine (mexitil) or 1-(2,6-dimethylphenoxy)-2-propanamine is a chiral non-selective voltage-gated sodium channel blocker, that is clinically used in its racemic form as an anti-myotonic, anti-arrhythmic, and analgesic agent (3-5). Mexiletine is a class Ib anti-arrhythmic drug that is the first choice drug in myotonia treatment with use-dependent behavior in inhibiting sodium currents in skeletal muscle fibers (6). By blocking sodium channels in skeletal muscle in a use-dependent manner, mexiletine inhibits the myotonic discharges of action potentials and favors muscle relaxation (6). In the other words, anti-myotonic drugs such as mexiletine facilitate the relaxation of skeletal muscles after electrical excitation. However, mexiletine as an important anti-myotonic drug has some disadvantages such as the high-therapeutic dose range, narrow therapeutic index, and serious side effects at cardiac and central nervous system levels upon chronic treatments (7, 8). Thus, the symptomatic therapy of myotonic syndromes needs to be improved for a selective treatment and reducing therapeutic dose range by means of a novel anti-myotonic agent. This goal can be achieved by common expensive new drug discovery researches or by low-cost clustering the established anti-myotonic drug units (i.e., mexiletine) on a suitable molecular scaffold as an alternative strategy for reducing the costs of researches. It should be noted that the clustering effect improves the bio-activity of the certain medication via impacting drug units in the cluster structure and consequently synergizing the pharmacological effects of the units.
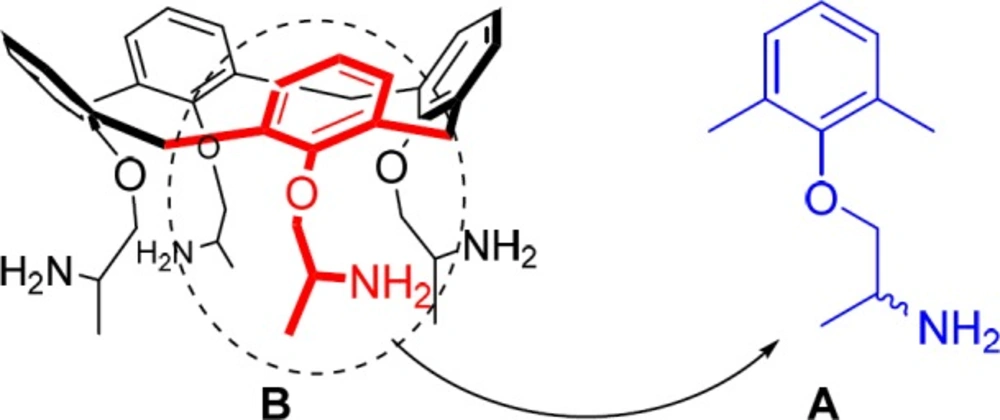
On the other hand, calixarenes, phenol-formaldehyde cyclic oligomers with three-dimensional structures, are suitable structures (without any notable in-vivo toxicity and immune responses) for designing and developing new drugs via clustering of single phenolic drug units such as mexiletine (9-14). So, in our chained studies for the synthesis of new calixdrugs (calixarene-based clusters of established therapeutic drug agents), in the present study, a novel calix[4]arene-based cluster of mexitil with chaliced shape has been reported and evaluated for its potentially enhanced bio-activity in inhibiting sodium currents in single skeletal muscle fibers in a use-dependent manner (anti-myotonic activity) with respect to its monomer mexiletine as reference drug (2). Based on calixarenes’ nomenclature, the chalice-shaped cluster of mexitil was innovatively named calixmexitil (2).
In summary, the main purpose of this paper is to describe the role of clustering effect, as an alternative and low-cost strategy for reducing the costs on drug discovery researches, in improvement of pharmaceutical properties and reducing side effects of mexiletine as the first choice drug in myotonia treatment via comparative study of drug affinity, use-dependent behavior, dose range of the cluster compound (calixmexitil) with regards to its monomer mexitil.
Experimental
Apparatuses and chemicals
The melting points of all compounds were recorded on Philip Harris C4954718 apparatus without calibration. IR spectra were determined on a Thermo Nicolet 610 Nexus FT-IR spectrometer in KBr disks. 1H (300 MHz) and 13C (75 MHz) NMR measurements were recorded on a Bruker AVANCE spectrometer in CDCl3 using TMS as the internal reference. Elemental analyses were performed using a Heraeus CHN-O-Rapido analyzer. Mass spectra were recorded on a JEOL-JMS 600 (FAB MS) instrument. Thin layer chromatography (TLC) analyses were carried out on silica gel plates. All chemicals were purchased from Merck, Sigma Aldrich, and Fluka Chemie (Tehran, Iran) and used as received by standard procedures. All reactions were carried out under a nitrogen or argon atmosphere.
Synthetic procedures
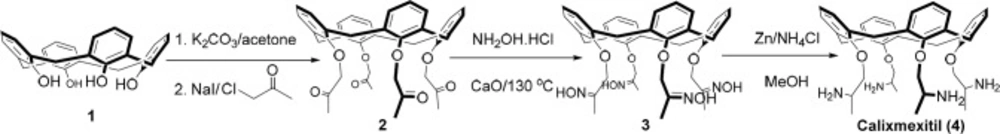
25,26,27,28-Tetrahydroxycalix[4]arene (1)
A slurry of p-t-butylcalix[4]arene (4 g, 6 mmol), phenol (2.82 g, 30 mmol) and AlCl3 (4.67, 35 mmol) was stirred in toluene (50 mL) at room temperature for 1 h in an inert atmosphere. The mixture was poured into 0.2 N HCl (80 mL), the organic phase was separated, and the toluene wan evaporated. Upon the addition of MeOH a precipitate formed, which was removed by filtration to give a solid. Recrystallization from MeOH-CHCl3 afforded compound 1 as colorless crystals.
Yield (1.96 g, 75%), mp: 314-316 °C. 1H NMR (300 MHz, CDCl3): δ 10.19 (s, 4H, OH), 7.22 (d, J = 8 Hz, 8H, Ar-Hm) 6.64 (t, J = 8Hz, 4H, Ar-Hp), 3.63-3.48 (bd, 8H, ArCH2Ar); 13C NMR (75 MHz, CDCl3): δ 148.4 (Ci), 129.0 (Cm), 128.2 (Co), 122.2 (Cp), 31.7 (ArCH2Ar).
25, 26, 27, 28- Tetrakis (methyl carbonylmethoxy)calix [4]arene (2)
To a stirred mixture of compound 1 (1.7 g, 4 mmol) and K2CO3 (4.35 g, 30 mmol) in acetone (50 mL) was added a solution of NaI (4.65 g, 30 mmol) and chloroacetone (2.5 mL, 30 mmol) in acetone (20 mL). The reaction mixture was heated to reflux under N2 atmosphere for 6 h. Then, it was cooled, filtered, and washed with fresh acetone (10 mL). After solvent evaporation, the residue was suspended in water (30 mL) at 60 °C and stirred for 2 h. Then the product was extracted into the dichloromethane (20 mL). Removal of solvent, left a pale-yellow solid which on recrystallization from acetone furnished the tetra-methyl ketone 2 as white crystals.
Yield (1.25 g, 48%), mp: 220-222 °C. IR (KBr, ν, cm − 1): 1731 (C = O). 1H NMR (300 MHz, CDCl3): δ 7.20 (d, J = 7.3 Hz, 8H, Ar-H m), 6.69 (t, J = 7.3 Hz, 4H, Ar-H p), 5.02 (d, J = 13 Hz, 4H, ArCH2Ar, Hax), 4.76 (s, 8H, ArO−CH2), 3.19 (d, J = 13 Hz, 4H, ArCH2Ar, Heq), 2.21 (s, 12H, CH3); 13C NMR (75 MHz, CDCl3): δ 205.2 (C=O), 153.1 (Ci), 136.2 (Co), 127.8 (Cm), 119.2 (Cp), 76.4 (ArOCH2), 31.7 (Me), 31.4 (ArCH2Ar). Anal. Calcd for C40H40O8: C, 74.06; H, 6.22. Found: C, 74.08; H, 6.19. FAB+MS m/z = 648.23 (M +).
| Compound | Tonic block IC50 (μM) | Phasic block IC50 (μM) | Tonic/Phasic ratio |
|---|---|---|---|
| Mexiletine | 89 | 31 | 3 |
| Calixmexitil | 28 | 3 | 9 |
25, 26, 27, 28- Tetrakis(methyl hydroxyiminomethoxy)calix [4]arene (3)
A mixture of compound 2 (1 g, 1.54 mmol) and calcium oxide (8.4 g, 0.15 mol) were heated to 130 °C in an oil bath for a few minutes. Then, hydroxylamine hydrochloride (3.12 g, 45 mmol) was added and the mixture was stirred with a magnetic stirrer in the presence of air for an hour. Then, the reaction mixture was mixed with ethyl acetate (50 mL), filtrate to remove CaO and mixed with water (30 mL) and extracted. The ethyl acetate solution was dried over Na2SO4. The solution was concentrated in vacuum and treated with dilute acetic acid (20 mL). The resulting yellow powder was filtered, washed with water, recrystallized from methanol and then from CH3OH/CHCl3 to give tetra oxime 3 as orange powder.
Yield (0.46 g, 42%), mp: 232-233 °C. IR (KBr, ν, cm − 1): 3572 (O-H), 1629 (C = N). 1H NMR (300 MHz, CDCl3): δ 12.48 (s, 4H, N-OH), 7.32 (d, J = 7.6 Hz, 8H, Ar-H m), 6.77 (t, J = 7.6 Hz, 4H, Ar-H p), 4.89 (d, J = 13.3 Hz, 4H, ArCH2Ar, Hax), 4.69 (s, 8H, ArO−CH2), 3.23 (d, J = 13.3 Hz, 4H, ArCH2Ar, Heq), 2.28 (s, 12H, CH3); 13C NMR (75 MHz, CDCl3): δ 160.9 (C=N), 152.8 (Ci), 138.2 (Co), 127.4 (Cm), 119.9 (Cp), 77.3 (ArOCH2), 32.1 (Me), 31.2 (ArCH2Ar). Anal. Calcd for C40H44N4O8: C, 67.78; H, 6.26; N, 7.90. Found: C, 67.76; H, 6.27; N, 7.93. FAB+MS m/z = 708.28 (M +).
Calixmexitil (4)
To a mixture of compound 3 (0.35 g, 0.5 mmol) in methanol (20 mL) was added NH4Cl (0.8 g, 15 mmol) and zinc powder (0.64g, 10 mmol). The mixture was stirred under reflux for 12 h. Then the reaction mixture was filtered and the filtrate was evaporated under vacuum and the residue was taken into chloroform (20 mL), washed with saturated NaCl solution (10 mL) and finally with water (10 mL). The organic layer was dried over anhydrous Na2SO4 and evaporation of the organic layer was followed by recrystallization of the residue in methanol/chloroform, to yield the final product as white crystals.
Yield (0.25 g, 77%), mp: 199-201 °C. IR (KBr, ν, cm − 1): 3306 (N-H). 1H NMR (300 MHz, CDCl3): δ 10.63 (bs, 8H, NH2), 7.48 (d, J = 7.3 Hz, 8H, Ar-H m), 6.84 (t, J = 7.3 Hz, 4H, Ar-H p), 4.96 (d, J = 13.7 Hz, 4H, ArCH2Ar, Hax), 4.53 (s, 8H, ArO−CH2), 3.44 (d, J = 13.7 Hz, 4H, ArCH2Ar, Heq), 2.32 (s, 12H, CH3); 13C NMR (75 MHz, CDCl3): δ 151.3 (Ci), 139.0 (Co), 126.8 (Cm), 118.7 (Cp), 76.8 (ArOCH2), 49.2 (C-N), 31.9 (Me), 31.3 (ArCH2Ar). Anal. Calcd for C40H52N4O4: C, 73.59; H, 8.03; N, 8.58. Found: C, 73.62; H, 8.01; N, 8.55. FAB+MS m/z = 652.44 (M +).
Biological Evaluations
Preparation and Solutions
For sodium current recordings, semitendinosus muscle fibers were perfused with the following “external” solution (mM): NaCl 77, choline-Cl 38, CaCl2 1.8, Na2HPO4 2.15, NaH2PO4 0.85; and dialyzed with the following “internal” solution (mM): CsF 105, MOPS 5, MgSO4 2, EGTA 5, Na2ATP 0.55 (pH) 7.2 with NaOH concentrated solution). Stock solutions of mexiletine and calixmexitil were prepared in physiological and/or “external” solutions. The final concentrations to be tested in-vitro were obtained by further diluting the stock solution as needed. DMSO at the highest concentration used (0.2%) was without effect on any of the parameters recorded.
Voltage Clamp Recordings of Sodium Current and Pulse Protocols
The voltage clamp recordings of sodium current were performed on single muscle fibers obtained with microsurgery from the ventral brunch of semitendinosus muscle of frog by means of the three vaseline gap voltage clamp technique as detailed elsewhere (15). After an equilibration time of 10 min, Na+ currents recordings were performed at 10 °C. The holding potential (hp) was -100 mV. The inward sodium traces were recorded using a voltage clamp amplifier based on that described by Hille and Campbell (16). The currents flowing in response to depolarizing command voltages were low pass filtered at 10 kHz visualized on an oscilloscope and sampled at 20 kHz. Maximal sodium currents were elicited with test pulses from the hp to -20 mV for 10 msec. Tonic block exerted by the test compounds was evaluated as percent reduction of the peak sodium current elicited by single test pulses. The evaluation of use-dependent block by the drugs was made by using a 10 Hz train of test pulses for a period of 30 sec and by normalizing the residual current at the end of this stimulation protocol with respect to that in the absence of drug.
Results and Discussion
Due to non-selective treatment of myotonia by high dose range of mexiletine and consequently, its serious side effects, it is necessary to improve the treatment by a novel anti-myotonic agent with amplified potency, low dose range, and enhanced use-dependent behavior (7, 8). Hypothetically, it can be possible via clustering the anti-myotonic drug mexiletine units on a suitable molecular backbone. On the other hand, due to the para-substituted phenolic structure of mexiletine, and considering the fact that calixarenes are the cyclic oligomer of phenols, the calix[4]arene as a suitable scaffold was chosen for clustering four impacted simple phenolic mexiletine units, in a chalice-shaped structure (calixmexitil), in order to improve both drug affinity and use-dependent behavior via clustering effect (2).
The synthetic route to calixmexitil 4 is depicted in Scheme 1. The synthetic route is similar (steps and reagents) to the synthetic pathway of the parent drug mexiletine. The synthetic strategy involves the grafting of four 2-aminopropoxy moieties on the lower rim of the calix[4]arene scaffold via reduction of the related tetra-oxime calix[4]arene 3 in the presence of zinc powder and ammonium chloride in methanol under reflux condition for 12 h (conversion of oxime to amine). The compound 3 was synthetized by a solvent-free reaction of tetra-methyl ketone calix[4]arene 2 with NH2OH.HCl salt in the presence of calcium oxide at 130 °C for an hour (conversion of carbonyl to oxime). The compound 2 was synthetized by the reaction of calix[4]arene 1 with chloroacetone in the presence of sodium iodide and potassium carbonate in acetone under reflux condition for 6 hours. All compounds’ structures were well characterized by NMR, FT-IR, and FAB-MS spectra and elemental analyses. Due to the zone of carbons’ chemical shifts (~ 31 ppm, 13C NMR) of methylene bridges in the structures of calixmexitil and its parent compounds 2 and 3, it can be concluded that the mentioned compounds have cone conformation (17).
In order to evaluate the potentially amplified biological activity of calixmexitil, it has been compared with its monomer (mexiletine) as reference drug in inhibiting sodium currents in single skeletal muscle fibers (Figure 1). For this purpose, they were evaluated for in-vitro activity as sodium channel blockers on single muscle fibers in a use-dependent manner.
The class IB anti-arrhythmic group of drugs (i.e., mexiletine) mainly act by preventing fast inward sodium channels from opening (sodium current inhibitor). However, this block has two components: The tonic block is time-insensitive and concentration-dependent (block of sodium channel at resting conditions evaluated during infrequent depolarizing pulses), whereas the phasic block (also called use-dependent, cumulative sodium current reduction by the drug at high stimulation frequency) depends on the rate of the depolarization. Phasic block (sodium current inhibition in use-dependent manner) can be considered as an indication of the anti-myotonic activity and it occurs in condition of high frequency of stimulation (18).
Concentrations of test channel blocker agents for half-maximal tonic and phasic block of sodium currents (IC50) in single skeletal muscle fiber are reported in Table 1.
Putative anti-myotonic activity of the test compound is the potency in phasic block producing, being indicated by the value of the phasic block IC50. Also, a test compound with much less value of phasic block IC50 is more potent than the rest of other test compounds in anti-myotonic activity.
As shown in the Table 1, calixmexitil (IC50 = 3 μM) is about 10-fold more potent than mexiletine (IC50 = 31 μM) in condition of high frequency of stimulation ([IC50 (mex phasic block)/IC50 (calix phasic block)] = 31/3 ~ 10) and consequently, it is more potent than mexiletine in considering as an anti-myotonic agent.
On the other hand, the ratio of IC50 (tonic block)/IC50 (phasic block) for a blocking agent indicates its use-dependency manner and this behavior would be better when the value of the ratio is high (18). As shown in the Table 1, calixmexitil is 3-fold more potent than the mexiletine (9/3 = 3) in the production of tonic/phasic blocks and consequently, it was more potent than mexiletine in exerting a use-dependent block.
In simple terms, the best anti-myotonic agent should be more potent in producing phasic block and use-dependent block in relation to the other test compounds.
Compared to mexitil, calixmexitil with less IC50 value for producing phasic block of sodium currents and more potency in exerting a use-dependent block is a “selective” anti-myotonic agent with “low-therapeutic dose range” and consequently, as mentioned in the introduction section (7, 8), hypothetically, via fewer side effects, it can be safer than mexiletine.
The enhanced pharmaceutical properties of calixmexitil are maybe attributed to clustering effect of four impacted mexiletine units and improved interaction of these units with the structure of the sodium channels in single muscle fibers.
In summary, the present work describes a low-cost drug discovery research for the synthesis of the first calixarene-based cluster (cyclic tetramer in chaliced shape) of mexiletine as a novel use-dependent sodium channel blocker with improved (10-fold) anti-myotonic activity and improved (3-fold) use-dependency behavior in comparison to its monomer, mexiletine as reference anti-myotonic drug. Compared to mexiletine, calixmexitil with improved potency in producing phasic block and use-dependent block is more “selective” anti-myotonic agent than mexitil in addition to possessing low-therapeutic dose range.