1. Background
Venous thromboembolism (VTE) includes deep vein thrombosis (DVT) and pulmonary embolism (PE). Many medically ill patients are at the risk of thrombosis formation in lower extremity veins defined as DVT. In addition, PE, which is a condition of pulmonary artery obstruction, is a serious life-threatening complication that could occur due to DVT formation and the embolization of the thrombus in hospitalized patients. Accordingly, it could be expected to prevent the incidence of PE and decrease mortality by preventing DVT. It is estimated that VTE accounts for 370,000 annual deaths in Europe (1).
Enoxaparin, a low-molecular-weight heparin (LMWH), is frequently used for thromboprophylaxis in conditions such as critically ill patients, orthopedic surgery, malignancy, and the like (2-4). The effects of subcutaneously injected enoxaparin on the coagulation pathway could be assessed by anti-factor Xa serum levels (5). Anti-Xa levels peak 3 - 5 hours after subcutaneous dosing (6), and the level reaches a steady-state after the 2nd to 4th doses (7).
Furthermore, enoxaparin could be administered in medical or surgical patients who are at risk of DVT and PE as an effective agent for prophylaxis. The desired peak anti-factor Xa level for prophylaxis against DVT/PE in medically ill populations is defined as 0.10 - 0.40 IU/mL (8, 9) and for surgical patients admitted to an intensive care unit (ICU) the peak level of 0.2 - 0.5 IU/mL has been aimed (10). Levels have a significant relationship with bleeding and thrombosis occurrence in surgical patients and are suggested to predict the clinical outcomes of patients (11).
In surgical patients and critical care, it has been demonstrated that enoxaparin administration by the standard prophylaxis dose of 40 mg could result in the sub-prophylactic levels of anti-Xa (12-14). Most studies in this area are conducted on critically ill, and surgery patients, and many dosing regimens for thromboprophylaxis have been proposed in this regard.
2. Objectives
In this study, it was hypothesized that higher doses would optimize thromboprophylaxis, and thus it was aimed to establish an enoxaparin optimal dose in adult non-critically ill patients hospitalized and in need of thromboprophylaxis based on anti-Xa levels.
3. Methods
3.1. Study Design
In this prospective randomized open-label clinical trial, 60 patients were enrolled from April 2018 to February 2019. These patients were diagnosed with an infective disease and admitted to the infectious ward in Loghman-Hakim tertiary medical center affiliated to Shahid Beheshti University of Medical Sciences, Tehran, Iran since they were candidates for receiving enoxaparin for thromboprophylaxis. It should be noted that the study was conducted in accordance with the Helsinki declaration. Prior to enrollment, written informed consent forms were obtained from all individuals. In addition, the study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences on 2/7/2018 (IR.SBMU.PHARMACY.REC.1397.069) and registered in the Iranian Registry on Clinical Trials (IRCT20130917014693N11).
Patients were included in the study if they were over 18 years old and needed enoxaparin administration to prevent the occurrence of thromboembolic events. On the other hand, the exclusion criteria included surgical patients, patients experiencing septic shock or sepsis-induced coagulopathy (15), acute kidney injury (an increase in the creatinine serum level of 0.3 mg/dL or more in 48 hours), and chronic kidney disease (with an estimated glomerular filtration rate (eGFR) of less than 30 mL/min or receiving renal replacement therapy), and diagnosis of disseminated intravascular coagulation (16). Other exclusion criteria included having a history of recent hemorrhage in the past month, undergoing an invasive process requiring enoxaparin discontinuation (eg, a lumbar puncture, pregnancy, and lactation), receiving therapeutic anticoagulation, malignancy, and thrombophilia, suffering from hyperbilirubinemia and antithrombin deficiency, having previous DVT or PE, having a body mass index (BMI) of 35 kg/m2 or more, a platelet count of 50000 cell/micL or less, the International normalized ratio of 1.5 or more, and the activated partial thromboplastin time of 2.5 times of a normal range or more.
3.2. Study Protocol
All enrolled patients were assessed for the risk of DVT occurrence based on the Padua risk assessment score, and patients with a score of 4 or more were included (17). Considering no contraindication for receiving enoxaparin, patients were randomly assigned to daily receive 40 and 60 mg enoxaparin equivalent to 4000 and 6000 international unit (IU) subcutaneously in the control and intervention groups, respectively. All doses of enoxaparin were manufactured in the pre-filled syringe and prepared for use in Alvand Pharmaceutical Company, Iran. After enrolment and ordering enoxaparin by the physician, full medical history was collected, followed by reviewing the systems of the patients by clinical pharmacy attending the ward and considering all exclusion criteria. Patients were also visited daily by infectious disease and clinical pharmacy attendings in accordance with reviewing the exclusion criteria. Patients were also examined for any signs and symptoms of thrombotic event or bleeding episode.
3.3. Sample Size and Randomization
The sample size was estimated by GPower® software, version 3.1. In general, 60 patients in two groups of 29 and 31 cases were estimated based on previous clinical data and considering the effect size of 0.8, α equivalent to 0.05, the type two error of 0.85, and equivalent group sizes. Block randomization method was used, through which 15 blocks consisting of four patients in each block were generated using online randomization website (Sealed Envelope Ltd.). In each block, two patients were allocated to the intervention group and two patients to the control group.
3.4. Data Collection
Demographic parameters for each patient (eg, age, gender, height, weight, and BMI) were collected in a prepared sheet. Moreover, the laboratory data of the electrolyte serum level, eGFR, liver enzymes and function, and coagulation monitoring were daily documented for each patient. In the prepared sheets, the cause of admission, underlying conditions, medication reconciliation, administered medication, and date and time of the first and fourth doses of enoxaparin were documented for each patient. The anti-Xa activity for each sample of patients was also documented in the sheets.
3.5. Anti-Xa Assay
The goal for anti-Xa was assumed to be the levels of 0.2 - 0.4 IU/mL in this study. The samples were centrifuged in the hospital laboratory and then analyzed by chromogenic assay for the anti-Xa plasma level via the STA Compact Max device. In this assay, excessive amounts of active factor X were added to the isolated plasma from each sample to bind to the LMWH. By adding chromogen to the sample and binding to the remaining factor Xa, the colored molecule was released, and the absorbent amount was measured by a spectrophotometer. The amount of absorption had a reverse correlation with anticoagulant concentration. Finally, anticoagulant concentration based on anti-Xa activity in units per milliliter was measured by drawing a standard curve.
3.6. Statistical Analysis
The Statistical Package for the Social Sciences (version 20.0) was used for statistical analyses. For data description, continuous variables were expressed as the mean ± standard deviation, and categorical ones were presented as numbers (percentage). To compare differences, paired sample t-test and chi-square test were utilized for quantitative and qualitative variables, respectively. A P-value < 0.05 was considered as statistically significant.
4. Results
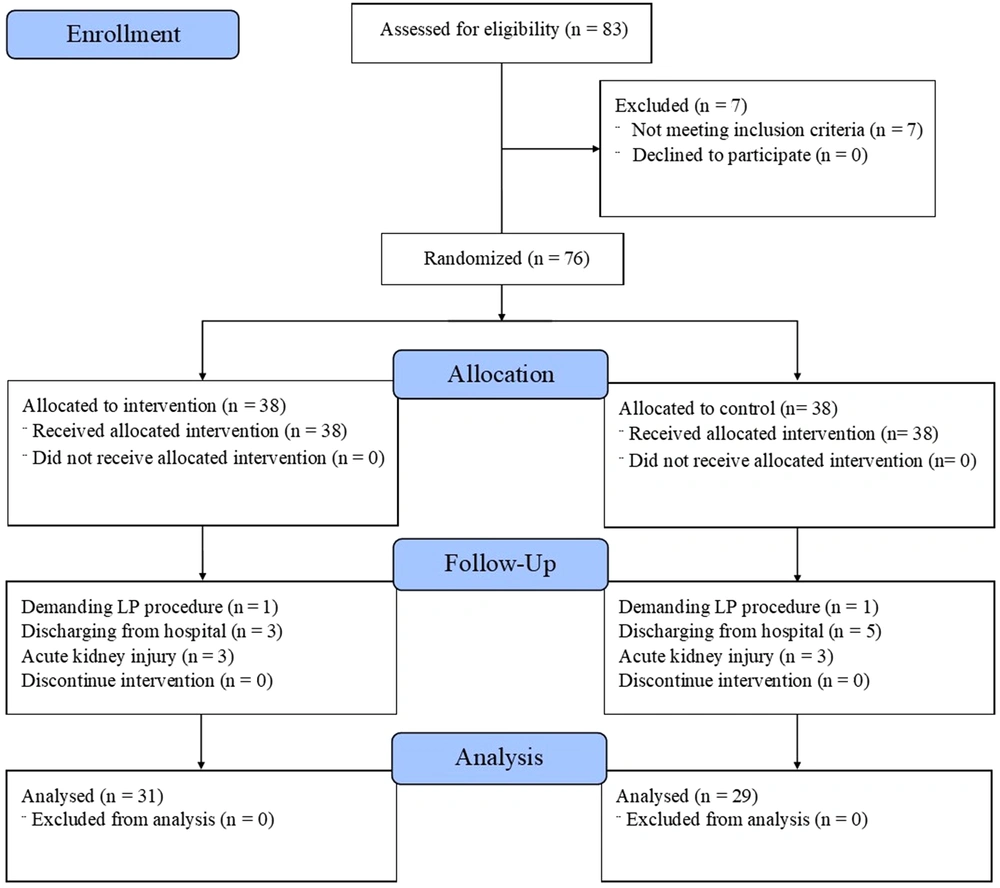
In this study, 83 patients were enrolled within ten months. The patients were admitted to the infectious ward in Loghman-Hakim Hospital and diagnosed with receiving enoxaparin to prevent thromboembolism. After assessing for eligibility, 76 patients were included and randomly divided into two equal groups (n = 38 each) to receive 40 or 60 mg enoxaparin daily by subcutaneous injection. The patients’ allocation for the trial is demonstrated in Figure 1.
The diagram illustrates the study enrollment and disposition of trial participants.
In general, 20 male and nine female patients with a mean age of 57.48 ± 23.87 years, as well as 20 male and 11 female patients with a mean age of 60 ± 19.30 were analyzed in the control and intervention groups, respectively. No significant differences were reported regarding age (P = 0.700) and gender (P = 0.788) distribution between the two groups.
Table 1 presents the demographic information and data on the infective cause of hospitalization (three most common), underlying conditions (three most common), and patients’ laboratory parameters in the two groups. Based on the results, no significant differences were observed with respect to liver enzyme elevation (P = 0.244) and electrolyte differences (P > 0.05). All the consumed medications were analyzed due to the potential for drug-drug interaction with enoxaparin. Table 1 provides data on the five most frequently consumed medications (ie, aspirin, clopidogrel, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and non-steroidal anti-inflammatory drugs) with potential interaction with enoxaparin.
| Variables | Intervention (N = 31) | Control (N = 29) | P-Value |
|---|---|---|---|
| Age (y) | 60.29 ± 19.30 | 57.48 ± 23.87 | 0.700 |
| Gender | 0.788 | ||
| Male | 20 (64.5) | 20 (69) | |
| Female | 11 (35.5) | 9 (31) | |
| Height (cm) | 167.94 ± 10.03 | 169.69 ± 9.00 | 0.761 |
| Weight (kg) | 68.35 ± 11.37 | 69.55 ± 10.64 | 0.640 |
| BMI (kg/m2) | 24.23 ± 3.30 | 24.21 ± 3.68 | 0.900 |
| Serum creatinine (mg/dL) | 1.00 ± 0.27 | 0.95 ± 0.28 | 0.357 |
| Urea (mg/dL) | 47.07 ± 29.03 | 39.64 ± 20.98 | 0.446 |
| Estimated glomerular filtration rate (mL/min/1.73 m2) | 62.87 ± 28.28 | 73.38 ± 32.73 | 0.171 |
| Underlying conditions (three most frequent) a | 0.735 | ||
| Without underlying disease | 10 | 12 | |
| Hypertension | 11 | 9 | |
| Diabetes | 1 | 1 | |
| Ischemic heart disease | 2 | 1 | |
| Infection cause of admission (three most frequent) b | 0.378 | ||
| Meningitis | 4 | 4 | |
| Encephalitis | 1 | 4 | |
| Community acquired pneumonia | 3 | 3 | |
| Medication with potential drug-drug interaction | |||
| Aspirin | 6 | 5 | 0.999 |
| Clopidogrel | 0 | 2 | 0.229 |
| Angiotensin converting enzyme inhibitors | 2 | 2 | 0.999 |
| Angiotensin receptor blockers | 6 | 3 | 0.474 |
| Non-steroidal inflammatory drugs | 0 | 1 | 0.483 |
| Coagulation profile | |||
| PT (sec) | 13.78 ± 1.27 | 13.79 ± 1.14 | 0.866 |
| INR | 1.20 ± 0.17 | 1.19 ± 0.13 | 0.781 |
| aPTT (sec) | 35.51 ± 5.89 | 34.95 ± 5.91 | 0.697 |
a Others: cerebrovascular accident, brain tumor, benign prostate hyperplasia, Alzheimer disease, Parkinson disease.
b Health-care associated pneumonia, pyelonephritis, cellulitis, diabetic foot infection, septic arthritis, fever of unknown origin, surgical site infection, gastroenteritis, brain abscess, bed sore, empyema, spondylodiscitis, brucellosis, chronic obstructive pulmonary disease exacerbation, chronic diarrhea.
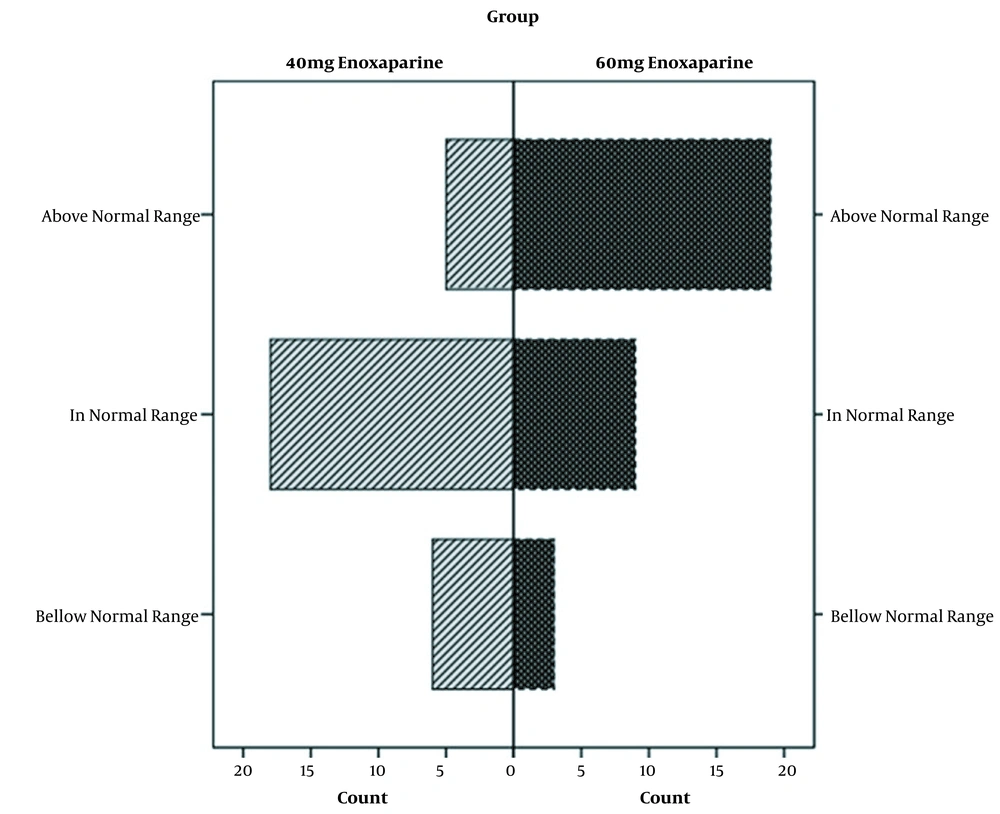
By analyzing anti-Xa plasma levels in two arms, differences reached a statistically significant level (P = 0.001). Furthermore, the mean anti-factor Xa was reported as 0.29 ± 0.13 and 0.44 ± 0.19 IU/mL in the control and intervention groups, respectively. The anti-Xa was in the range of less than, or more than the target value in 18 (62.1%), six (20.7%), and five (17.2%) patients in the control group, respectively. In the intervention group, the level of anti-Xa was in the range of, less than, and more than the target value in nine (29%), three (9.7%), and 19 (61.3%) patients, respectively.
All patients were monitored for major bleeding episodes and thromboembolic events during the study. None of the patients showed any signs and symptoms of these safety outcomes. Moreover, no bleeding occurred in the intervention group considering higher anti-factor Xa. Eventually, no thromboembolic events happened in the control group with lower anti-factor Xa (Figure 2)
5. Discussion
Thromboembolic events could occur and affect any venous circulation in the body. DVT is a fairly common disease, and its complications (eg, PE) are associated with a reduced survival rate and increase in related health care costs (18). Additionally, VTE is considered multifactorial. By considering the annual mortality rate of 30000 individuals, more people are dying from VTE than breast cancer or human immunodeficiency-related virus (HIV) (19).
The major risk factor for thromboembolism could be divided into extrinsic (eg, major surgery, hospitalization and bed rest, trauma, pregnancy, postpartum condition, and hormone replacement therapy) and intrinsic (eg, malignancy, obesity, and hyper-coagulopathy conditions) categories (20). In hospitalized patients, the risk of VTE could be even 130 times more than in non-hospitalized patients (21-23). Furthermore, the specific pharmacokinetic properties of enoxaparin made this agent the medication of choice for thromboprophylaxis. In addition, a longer half-life, predictable bioavailability, and methods for monitoring its anticoagulation effect made this agent an interesting option.
Enoxaparin, as the thromboprophylaxis agent of choice by the American Society of Hematology (ASH) and the American College of Chest Physicians (CHEST), is now being used frequently (24). It is recommended that anticoagulation should be assessed by enoxaparin in patients with obesity and renal impairments, as well as elderly and pediatric patients (25). In one study, it was demonstrated that lower anti-factor Xa is more prevalent in traumatic patients compared to previous estimates (26). In patients receiving usual doses of thromboprophylaxis with enoxaparin, there is still the risk of failure, which could be because of insufficient enoxaparin doses received by patients (27). Specifically, it has been demonstrated that surgical patients are at increased risk of therapy failure. Levine et al. evaluated the relation between the levels of anti-Xa and VTE or bleeding episodes in 163 post-orthopedic surgery patients receiving thromboprophylaxis by enoxaparin; they found that anti-Xa lower than 0.1 IU/mL was related to more thromboembolic events (11). In another study, it was demonstrated that 40 mg enoxaparin once-daily administration produced inadequate thromboprophylaxis in most patients undergoing thoracic surgery (28). This phenomenon was observed in medical patients. In the study by Ramos-Esquivel and Salazar-Sanchez, sub-therapeutic levels were observed in one-third of the patients (29). Also, attending physicians of the infectious disease ward administered enoxaparin 60 mg daily in some patients. An appropriate dose of enoxaparin leading to enough anti-Xa levels could prevent the occurrence of thromboembolic episodes. In our study, the goal of anti-Xa was considered 0.2 - 0.4 IU/mL, and no thromboembolic or bleeding episodes happened in the two groups. In addition, patients in the two groups did not differ in terms of demographic and laboratory data. Based on the results, levels less than the target value for anti-Xa (< 0.2 IU/mL), which could be associated with more thromboembolic events, were reported in the control group more frequently compared to the intervention group (20.7% vs. 9.7%). However, patients with an anti-Xa level more than the upper limit for thromboprophylaxis (> 0.4 IU/mL) were more frequent in the intervention group than the control group (61.3% vs. 17.2%), which is related to more bleeding risk. More importantly, patients receiving 40 mg of daily enoxaparin showed a normal anti-Xa level for thromboprophylaxis more frequently compared to those patients receiving 60 mg enoxaparin daily (62.1% vs. 29%).
Robinson et al. (13) found that enoxaparin increased thromboprophylaxis doses in patients admitted to the ICU. The anti-Xa level was significantly different in patients receiving enoxaparin by different daily doses of 40, 50, 60, or 70 mg by subcutaneous injection. In this study, it was shown that 60 mg enoxaparin was more consistent with patients with the target level of anti-Xa, and subcutaneous injection of 40 mg enoxaparin showed insufficient anti-Xa levels (11). Patients with critically ill conditions such as hyperdynamic conditions, edema, and a shock state could differently respond to our medications. Furthermore, vasopressor use in these patients could affect the pharmacokinetics of medication, which is administered by a subcutaneous route. Accordingly, it is reasonable to expect that critically ill patients have less anti-factor Xa levels compared to non-ICU admitted patients with the same dose of enoxaparin (13, 27).
Safety consideration in enoxaparin use is another important issue when applying higher doses of prophylaxis. Higher doses of anticoagulants are related to higher bleeding episodes. Pannucci et al. demonstrated that 40 mg of enoxaparin two times daily was simultaneously associated with more target levels of anti-factor Xa and more bleeding episodes (27).
Bleeding episodes and thromboembolic events are two ends of the spectrum of anticoagulant use effects, both of which could be dangerous. Nonetheless, many other important factors could affect the anti-Xa level in enoxaparin consumption, including weight, BMI, renal function, other demographic parameters, along with enoxaparin itself and anti-factor Xa assay. Patients with a higher BMI need higher doses of enoxaparin for reaching the anti-factor Xa goal. Furthermore, renal impairments could affect enoxaparin pharmacokinetics. Thus, patients with an eGFR of less than 30 mL/min need adjusted enoxaparin doses for anticoagulation in the prophylaxis of DVT/PE (30, 31). Various brands of enoxaparin, because of different sources of production, could lead to a wide variety of medication potency levels. Therefore, it could be recommended that the pharmacokinetic studies of each medication based on the anti-factor Xa level should be performed for each source of enoxaparin.
The present study had some limitations. To detect safety outcomes, it could be recommended to use a larger sample size, and the sample size should be determined based on the efficacy of enoxaparin. Moreover, considering that the anti-factor Xa assay is a high-cost laboratory exam, it was impossible to increase our study population. Additionally, a longer duration of enoxaparin consumption is necessary for assessing the safety profile of its different doses.
5.1. Conclusions
The results of our study showed that the subcutaneous injection of 40 mg daily provided sufficient anti-Xa levels in most non-critically ill patients who needed enoxaparin administration for thromboprophylaxis. In addition, using enoxaparin 60 mg daily provided anti-Xa levels higher than the upper limit of the thromboprophylaxis range in most patients. It is important to consider the safety of higher levels of anti-factor Xa. Finally, none of the patients in our study demonstrated any signs and symptoms of bleeding, although using higher doses of enoxaparin could lead to serious problems when used for a longer duration, which needs further long-term studies.