1. Background
The importance of antioxidants is related to their role in reducing cellular damage during biological oxidation (1).
The free radicals scavenging properties of antioxidants help to prevent or delay oxidation. Although free radicals are products of cellular metabolism, an excessive number of free radicals in cells leads to oxidative stress, which eventually causes damage to biomacromolecules such as proteins, lipids, and nucleic acids (2-4). Hence, antioxidants are promising factors in preventive medicine to reduce oxidative side effects besides playing an important role in the food and cosmetics production industry (5, 6).
Synthetic antioxidants have some limitations, such as the difficulty of their synthesis (1) and some toxicity effects (5, 7). Regarding to them, natural sources of antioxidants are really considerable.
Medicinal plants are great sources of antioxidants and phenolic compounds that are mainly responsible for these effects (8). Their structure is the primary cause of their antioxidant properties (9-11). Two chemical parts of phenolic compounds play a critical role in free radical scavenging: phenolic hydroxyl groups and the hydrocarbon backbone (10). Shreds of evidence confirmed a strong connection between the content of phenolic compounds and antioxidant activities (9, 10, 12-14).
Various species of the Rosa gender are broadly distributed across Europe, Asia, North America, and the Middle East. Rose hips are related to the pseudo fruits of the Rosa spp, particularly Rosa canina L. (Rosaceae). They are used in the pharmaceutical, food processing, and cosmetic industries. Concerning their health benefits, rose hips have attracted much attention in recent studies (1, 15).
The use of R. canina dates back to the time of Hippocrates in ancient Greek. Also, R. canina was recommended to treat headaches and some neural and gastrointestinal diseases in the Canon of Medicine written by Avicenna. So, it has been famous in traditional Persian medicine. Moreover, Aghili suggested it as a tonic for the heart and brain and for treating some hepatic disorders (16, 17).
In central European countries such as Germany and Hungary, these fruits are commonly used to treat colds and influenza (1).
The fruit extract of Rosa canina has been shown to have an anti-inflammatory effect. Moreover, in vitro studies have shown that galactolipid of rose hip showed neutrophil chemotaxis effects (18). Oral administration of the Rosa canina fruit extract improves blood glucose, reduces necrosis in islet cells, and regenerates pancreatic islets (17, 19). Moreover, the inhibition of α -amylase has been reported to be another mechanism of this fruit to control diabetes (20). Previous studies recommend the administration of this fruit to reduce pain in osteoarthritic patients (21). Moreover, this fruit extract has a protective effect on hepatotoxicity induced by carbon tetrachloride (22).
The different biological advantages of the rose hip fruits are related to their phytochemichal component (1). The fruit contains vitamins, especially ascorbic acid (vitamin C), minerals, sugars, fatty acids (23), and other phytochemical compounds (24). This content depends on the geographical location or/ and variety of the plants (1).
There are several ways to enrich the bioactive compounds, such as liquid-liquid extraction, counter current chromatography, and column chromatography. One of the disadvantages of these methods is applying toxic organic solvents; another drawback is the long steps and high cost of materials. Based on the types of solvents and short steps, Amberlite resins with variant affinities for different classes of compounds could be considered a rapid, low cost, and safe way compared to the other methods. In this study, the polyphenol-rich fraction of Rose hip fruits was prepared using XAD-7 chromatography. Furthermore, the antioxidant activity, ascorbic acid content, XO inhibition, and protective effects on isolated human lymphocytes of the total extract and Amberlite enriched phenolic fraction were measured.
2. Methods
2.1. Plant Material
Rosa canina fruits were manually harvested during August and September 2105 in Sepidan (Fars Province, Iran). They were identified by Ms. Sedigheh Khademian in the herbarium of medicinal plants, Shiraz University of Medical Sciences, Shiraz, Iran (voucher no. 2660).
2.2. Preparation of Phenolic Enriched Fraction (PEF)
The fruits were freeze-dried by freeze-dryer and grounded into a powder; then, this powder was percolated with ethanol for 48 h and concentrated in a rotary evaporator, then gummy material was produced by speed vacuum. Then, it was suspended in trifluoroacetic acid (TFA), filtered, and poured into a separate funnel containing ethyl acetate. This extraction procedure by adding ethyl acetate was repeated in triplicate. Eventually, the aqueous phase was collected, freeze-dried, and loaded on the column (2.5 × 45 cm) containing Amberlite XAD-7 stationary phase. It was eluted with distilled water: TFA, followed by methanol: TFA. The fraction eluted by methanol: TFA was a phenolic enriched fraction (PEF) (yield: 20%) (25).
2.3. HPLC Analysis of Ascorbic Acid
PEF was analyzed for determination of ascorbic acid content by an HPLC system (Shimadzu Corporation, Kyoto, Japan), coupled with a Eurospher 100, C18 column (5 µm, 250 × 4.6 mm, Knauer) and UV-Visible detection at 245 nm. The mobile phase consisted of an isocratic elution procedure employing a mixture of NaH2PO4-acetonitrile (93:7) with a flow rate of 1 mL min-1. The calibration curve and quantitative evaluations were accomplished using standard ascorbic acid. The calibration curve was plotted for the concentration range of 2.34 - 37.5 µg mL-1 based on a 5-point calibration. Quantity determination was performed by comparing the chromatographic peak area with that of the external standard.
2.4. Determination of Total Phenolic, Flavonoid, and Anthocyanin Content
Folin-Ciocalteu as a reagent and gallic acid (GA) as standard were used to measure total phenolic compounds in PEF. Each test was done in triplicate.
A standard calibration curve was established using different concentrations of GA. Total phenolic was reported as GA equivalents (mg) per g of sample (26).
The flavonoid content of each sample was quantified using a colorimetric assay. The different concentrations of quercetin were used as a standard to prepare the calibration curve. The flavonoid content was shown as quercetin equivalents (mg) per g of sample. The results were presented as mean ± standard deviation (SD) for triplicate (27). The anthocyanin content was measured based on the previously described method (28).
2.5. DPPH Method
The radical scavenging ability of PEF was measured by DPPH assay (29).
Briefly, the mixture of 200 µL of DPPH solution and 20 µL of sample solutions with different ranges of concentration were prepared. The microplate was incubated for 30 min at 25°C, and the absorbance was measured at 492 nm. The concentrations of the samples which were able to scavenge 50% of the DPPH free radicals were defined as IC50 content. In this assay, quercetin was applied as a standard.
2.6. Nitric Oxide Free Radical Scavenging Assay
In this assay, the sodium nitroprusside and sample mixture were incubated at 27°C for 150 min. After incubation, 100 µL of Griess reagent was added to each sample and then incubated at room temperature. The absorbance was measured at 542 nm (30).
2.7. FRAP (Ferric-Reducing Antioxidant Power) Assay
The FRAP reagent contains different solutions, including TPTZ solution in HCl, FeCl3, and acetate buffer. The fresh FRAP reagent was prepared and incubated at 37°C. The mixture of FRAP reagent (180 µL) and each sample (20 µL) was prepared. The absorbance of the mixture was evaluated at 593 nm after 10 min incubation at 37°C (28).
2.8. ABTS+• Radical-Scavenging Assay
ABTS+• was produced by mixing 5 mL ABTS diammonium salt aqueous solution with 5 mL ammonium persulfate solution. Before using the mixture, it must be kept in the dark for 16 h (at room temperature). Before analysis, the ABTS solution was diluted with ethanol to obtain an absorbance of 0.70 ± 0.05 at a wavelength of 734 nm. ABTS radical solution (200 μL) was added to 20 μL of the sample. After 15 minutes, the absorbance was evaluated at 734 nm (31).
2.9. Ferric Thiocyanate (FTC) Method
A mixture containing a sample in ethanol, 4.1 mL of linoleic acid in ethanol, 8.0 mL phosphate buffer, and 3.9 mL of water was prepared and kept in a dark place (40°C).
9.7 mL of ethanol and 0.1 mL of ammonium thiocyanate were added to the mentioned mixture. Then 0.1 mL of ferrous chloride in HCl was added to the reaction mixture. The absorbance of the red color was measured at 500 nm each 24 h until the day after absorbance of control reached the maximum.
2.10. Oxygen Radical Absorbance Capacity Assay (ORAC)
The procedure was carried out using the polar star omega device according to the defined method. Briefly, fluorescein solution AAPH and sample or phosphate buffer as the blank were mixed. The ORAC values were defined as Trolox equivalents (TE) (25).
2.11. Antioxidant Assay for Cellular Antioxidant Activity (CAA)
HepG2 cells (6 × 104 cells/well) were incubated overnight.
Firstly, the medium was discarded, and then each well was washed with PBS triplicate; the wells were treated with RPMI containing DCFH-DA and different concentrations of quercetin as a standard or sample. The microplate was incubated at 37°C. Then the liquid was discarded, and the cells were washed by PBS in the final step, AAPH (free radical initiator) was applied to the cells, and the plate was read by the polar star omega device (32).
2.12. Xanthine Oxidase Assay
XO activity was analyzed based on the protocol of the oxidase activity assay Kit (Sigma-Aldrich brand). The xanthine oxidase activity of the sample and 100 µg/mL of vitamin C were measured (25).
2.13. In Vitro Comet Assay Experiments with Human Lymphocytes
All procedures in this study were conducted in line with the declaration of Helsinki.
Fresh lymphocytes were separated from healthy volunteer blood (25 - 30 years), and trypan blue dye was used to determine cell viability (33). The comet assay was performed based on the method of Singh et al. (34). The cells were exposed to various concentrations of samples and 50 µM H2O2 concurrently, in the dark at 4°C (20 min) to prevent repair of the induced DNA damages. H2O2 was used as a positive control.
The cells were harvested and centrifuged and then washed with PBS. The cell pellets were mixed with low melting point agarose followed by spreading on a fully frosted microscopic slide precoated with normal melting agarose, covered with a cover slip. The slides were immersed in a freshly prepared cold lysing solution. Then, the samples were placed in the electrophoretic tank with an alkaline solution (pH 13), and electrophoresis was performed.
Then slides were placed in a tank vertically and washed with neutralizing buffer. Finally, propidium iodide was used to stain the slides (20 µg/mL); the stain was dispensed directly onto the slides and covered with a coverslip. The slides were analyzed by an Olympus-BX61 fluorescent microscope. Each test was performed minimum 3e times. Fifty selected cells of each slide were analyzed by Casplab software.
2.14. Statistical Analysis
Comet assay results were analyzed by ANOVA and Tukey's posttest SPSS software. Values were expressed as mean ± SD of tree experiments. Statistical significance was determined at P-value < 0.05.
3. Results and Discussion
3.1. Phenol Content
The results of the phenol content of Rosa canina (R. canina) are shown in Table 1.
| Sample | Total Phenolic (mg GAE/1g Extract) | Total Flavonoids (mg QE/1g Extract) | Total Anthocyanins (mg/g-1) |
|---|---|---|---|
| Rosa canina extract | 186.71 ± 0.72 | 73.63 ± 0.42 | 0.11 ± 0.003 |
| Phenolic rich fraction | 325.02 ± 0.13 | 164.20 ± 0.30 | 1.8 ± 0.19 |
Abbreviation: QE, quercetin equivalent.
Our study revealed a significant increase in total phenol, flavonoid, and anthocyanin content of PEF compared to extract (P < 0.0001).
The phenol content of PEF of R. canina was significantly higher than the reported ones in the literature. Fattahi et al. reported 1.99 mg GAE/g (GA equivalent per gram) (13). Nowak and Gawlik-Dziki reported 9.9 mg GAE/dry plant and 8.18 mg GAE/g fresh fruits (35). In a study conveyed by Ercisli phenol content was reported as 96 mg/g dry fruit (36).
In another study, the total phenol content in three extracts (water, 50% v/v ethanol, 70% v/v ethanol) obtained from dry rose hip fruits was 55.4 - 69.4 mg GA/g dry fruit.
The highest total phenol content belonged to 50% (v/v) ethanol extracts, while the lowest reported value was related to water extract (27). The phenol content in the rose hip extract ranged from 3.26 to 5.75 mg GA/g in different varieties of this plant from the spontaneous flora of Transylvania (37).
A comparison of R. canina hips ecotypes in different regions of Iran showed significant differences between ecotypes in terms of total phenol contents. These hips contained 73.39 - 104.70 mg GA/g dry fruits (38). Total phenol contents in 25 rose hip fruit types selected from Bolu-Turkey were in the range of 20.12 - 32.2 mg GAE/g (39).
The total flavonoid content of the PEF increased significantly compared to the extract (P < 0.0001). Previous studies reported different ranges of flavonoid concentrations, such as 1.04 mg QE/g (mg quercetin equivalent (QE)/g) fresh weight for methanol extracts (40). 0.02 mg QE/g methanolic extract (13) and 0.11 - 0.41 mg RE/mL (Rutin Equivalent/mL) in R. canina hips extract from Tunisia (41). In R. canina L. biotypes from spontaneous flora of Transylvania, the total flavonoid content was reported to be in the range of 1.01 - 1.63 mg QE/g (37).
These studies describe the rose hip as a rich source of phenol compounds. The identified flavan-3-ols in the rose hip crude extract are catechin, galloyl derivates, and catechin hexoside (42, 43).
Other significant phenolic compounds include rutin, quercetin galactoside, quercetin glucoside, proanthocyanidine (42), GA, and caffeic acid (1). Phenolic acids were reported by Cunja et al. in rose hips using HPLC/MS, including gallic and ellagic acid derivate, coumaroylquinic acid, and p-cumaric acid. Variation in the content of phenolic acid derivatives depends on the rose hip maturation level and frost time (44).
Table 1 shows that the total anthocyanin content in the PEF is about 1.8 mg/g, which increased significantly compared to the extract (P < 0.0001). The reported anthocyanin content in R. canina ecotypes in the central region of Iran was in the range of 74 - 102 μmol/g fruit weight (μmol/g FW).
The crude rose hips extract analysis using HPLC DAD-ESI/MS revealed that this extract contains only cyanidin-3-O- glucoside (42). This compound's content is higher in well-matured rose hips than in its fruits in its early stages (44).
In brief, the significant increase in the total phenol, flavonoids, and anthocyanin content of the fraction compared to the extract could mean that the Amberlite XAD-7 chromatography adsorbent removed nonaromatic polar compounds such as polysaccharides and protein from the extract and increased the concentration of phenol compounds (23).
3.2. Ascorbic Acid Analysis
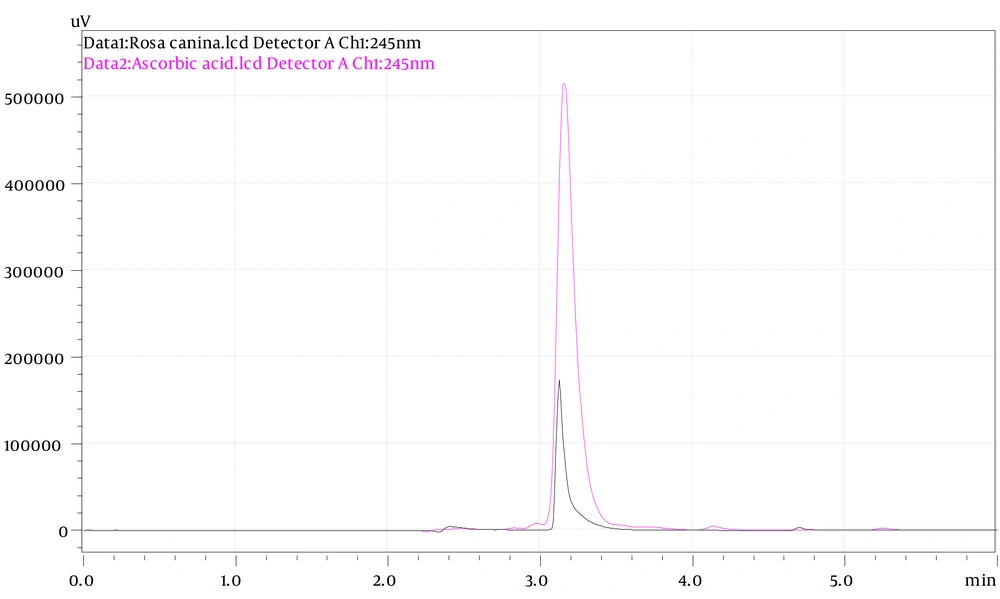
The HPLC-UV system was used to analyze the concentration of ascorbic acid in rose hips.
Ascorbic acid was used as a standard (R2 = 0.99).
Figure 1 shows the HPLC chromatograms of the ascorbic acid from rose hips and standard.
The concentration of ascorbic acid in the PEF was 7.51 ± 0.26 mg/g.
The recent analysis of R. canina ecotypes in the central part of Iran showed that the highest ascorbic acid content was 13.58 mg/g FW (fruit weight) from Zarneh and the lower content (5.18 mg/g FW) was from Miyankish. This content was 7.17 mg/g FW in R. canina from the Aghcheh region, which agrees with our results (38). The lowest reported ascorbic acid concentration (0.4 mg/g dry) belonged to a sample from Iran (45).
In different agro-climatic regions of Turkey, the reported ascorbic acid concentration was 1.06 - 27.12 mg/g of fresh pulp in different rose species (37).
The average amounts of ascorbic acid in various Romanian rose hips pulp were between 3.60 - 1.12 mg/g (37). The ascorbic acid concentration in dry Rosa canina fruits from Spain was 0.10 mg/g (1).
The ascorbic acid content of rose hip fruits from southwest Bulgaria was extracted using a different solvent. This content was 0.89 mg/g dw for 50% ethanol extract, 24.04 mg/g dw for 70% dw ethanol extract, and 23.40.2 mg/100 g dw for distilled water (27).
The ascorbic acid content depends on different environmental and geographical factors (35, 37).
3.3. Antioxidant Assay
The radical scavenging activity of the extract and fraction of R. canina was measured using different methods. The results are shown in Table 2. The antioxidant activity of the extract amplified significantly compared to the fraction in DPPH, nitric oxide, and ABTS assays (P < 0.001). Furthermore, the reducing ability of the fraction to convert Fe3+ to Fe2+ was measured using FRAP assay.
| Sample | DPPH (IC50, µg/mL) | Nitric Oxide Scavenging % (200 µg/mL) | FRAP (IC50, µg/mL) | ABTS (IC50, µg/mL) |
|---|---|---|---|---|
| Extract | 476 ± 0.50 | 53.22 ± 1.25 | 39 ± 0.03 | 720 ± 1.2 |
| Phenolic rich fraction | 57 ± 0.57 | 91.16 ± 0.69 | 7 ± 0.01 | 58 ± 0.5 |
| Quercetin | 26.51 ± 0.06 | - | 8.69 ±0.03 | 25.64 ± 0.02 |
Abbreviation: FRAP, ferric-reducing antioxidant power.
Previous studies have shown that the fruit of R. canina is a powerful source of antioxidants (1).
Two other antioxidant assays, namely ORAC and CCA, were done for the fraction of R. canina. In ORAC assay, the ability of the fraction to participate in hydrogen atom transfer was determined (17). According to the Trolox calibration curve, the TEs for ORAC assay were 0.72 ± 0. 021 and 1.05 ± 0.04 for quercetin and the PEF, respectively.
CAA assay help to predict the antioxidant activity of the sample in cellular conditions. The EC50 values in this test were 55.51 ± 0.21 and 82.19 ± 0.42 µg/mL for quercetin and the PEF, respectively. This means that the PEF could inhibit the oxidation of DCFH to DCF in the cells (19).
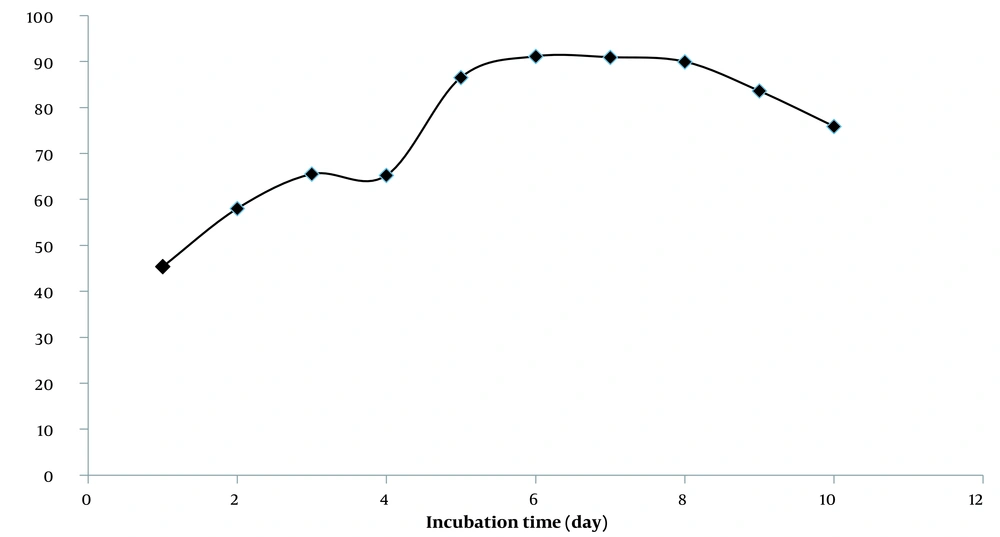
The lipid peroxidation inhibition study of PEF showed that the fraction could inhibit lipid peroxidation for six days (Figure 2). These results and some previous research described the hydroalcoholic extract of rose hips as a free radical terminator and inhibitor of lipid oxidation (46).
The more antioxidant ability of PEF could be related to the higher concentration of the phenolic compounds compared to the extract, as mentioned in the previous section. The total number and position of the hydroxyl functional groups in the phenolic compounds are responsible for reducing oxidizing free radicals (47). Moreover, chelating metal ions, scavenging ROS, and inhibiting oxidases are other mechanisms of phenol compounds to inhibit free radical formations (47).
3.4. Xanthine Oxidase Activity
XO has a role in oxidative stress and oxidative damage. This enzyme participates in purine catabolism and uses O2 as an electron acceptor. Therefore, O2•- and H2O2 are products of its reactions. Additionally, XO plays a key role in developing hyperuricemia, gout, cardiovascular diseases, hypertension, and metabolic syndrome (48).
In recent decades, novel natural or synthetic XO inhibitors have been screened (23). Phenolic compounds are introduced as potent inhibitors of the XO enzyme (24). The result of the XO inhibition activity of the PEF is 95.4 ± 0.001% and is 90.2 6 ± 0.003% for ascorbic acid.
3.5. Comet Assay Analysis
Reactive oxygen species (ROS) are products of different cellular or environmental pathways. ROS and DNA interaction can lead to DNA oxidative damages that are severely related to the development of aging, cancer, and other diseases (49).
Comet assay was done to measure the probable protective effect of R. canina fraction on DNA damage induced by H2O2 in leucocytes.
In protective studies, cells were exposed to ROS producers such as H2O2 and PEF simultaneously, and DNA damages were analyzed by comet assay.
Antioxidant potency is inversely related to DNA breaks (24). Comet assay is reliable for measuring oxidative DNA damage (28, 32, 50).
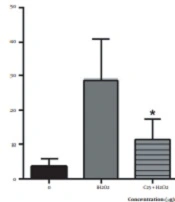
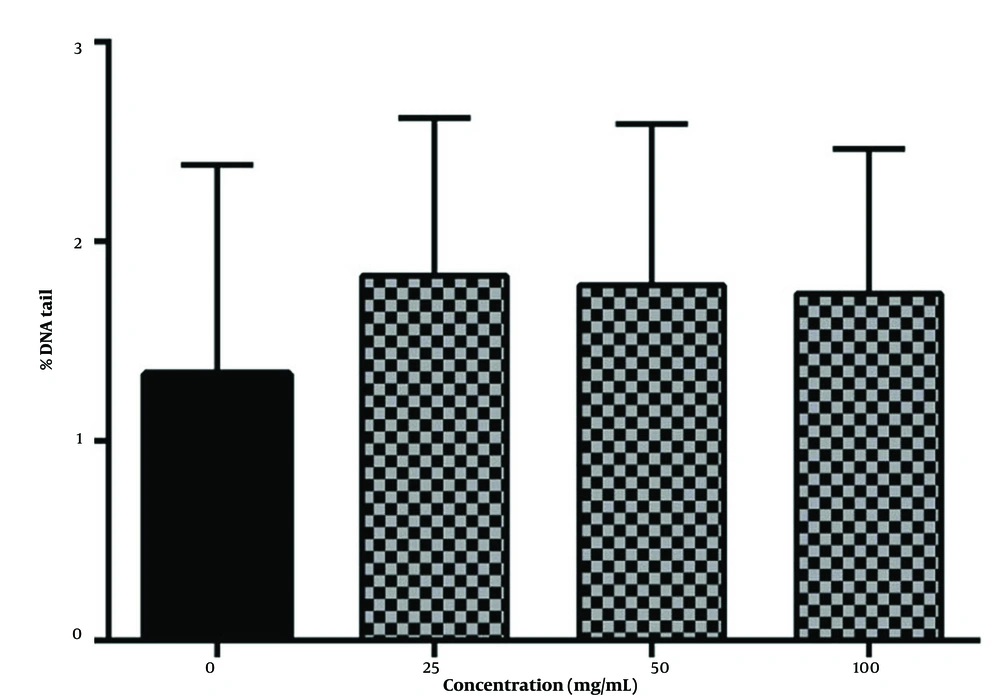
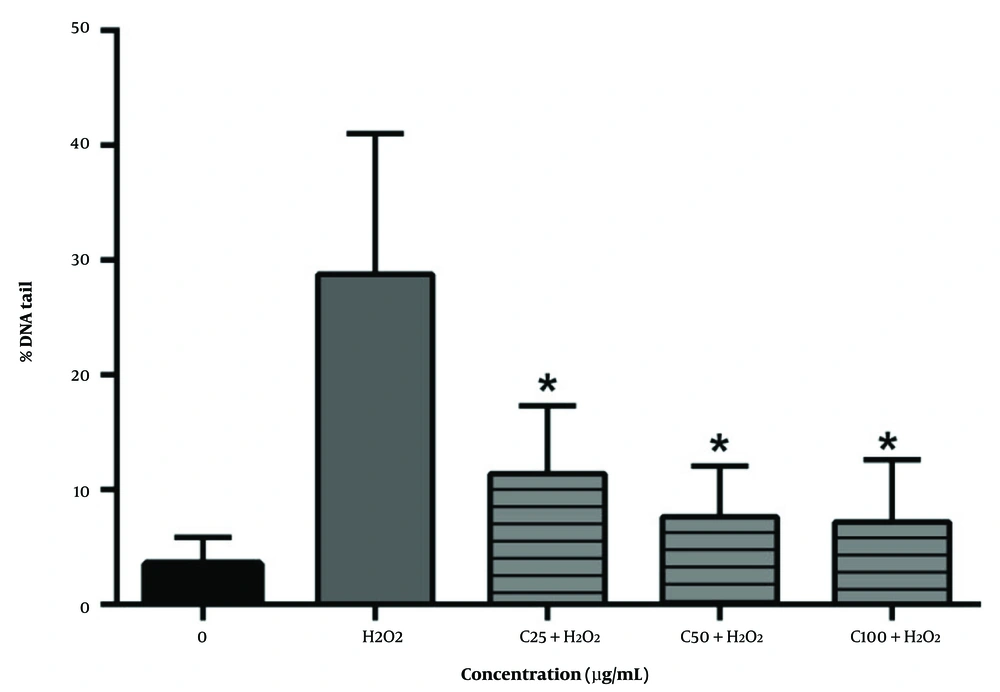
Lymphocytes exposed to 25, 50, and 100 µg/mL concentrations of fraction and H2O2 concurrently show a low level of DNA damage (P < 0.05), while H2O2 solely causes significant DNA strand breaks (Figure 3).
These results indicate that the presence of phenol compounds provokes oxidative DNA damage; this is supported by the antioxidant activity of the fraction measured by different methods and the high amount of phenolic compounds and ascorbic acid in this fraction (Figure 4).
It appears that phenol compounds scavenge radicals before they induce any DNA damage. Hydrogen atoms from their structure can remove hydroxyl radicals generated from H2O2 (47, 50). Also, the ortho dihydroxy group in their structure can conjugate the transition metal such as Fe and Cu to inhibit the Fenton reaction (25).
In summary, an integrated extraction-adsorption by Amberlite XAD-7 ion exchange chromatography provides a process to enrich phenolic compounds in a single procedure.
Also, the energy consumption of this chromatography method during extraction is really less than other equipment. Moreover, solvent extraction is non-selective, and other compounds were co-extracted with polyphenols.
Compared to our study, the total polyphenol and anthocyanin of chokeberries were recovered using Amberlite XAD 7HP. Eighty-two percent of total chokeberry phenolics and 92% of its anthocyanins were separated (51). In another report, this technique has been designed to recover antioxidant polyphenols from Aronia melanocarpa (51) and Scutellariae barbatae herba (52).
4. Conclusions
The present study recommends Amberlite XAD-7 as a chromatography adsorbent in the separation process to enrich the phenol contents. Compared to its total extract, the polyphenol-enriched fraction of Rosa canina fruits was a potent source of antioxidants. It showed xanthine oxidase inhibitory activity and DNA damage protection, probably due to the high amount of phenol compounds and ascorbic acid.