1. Background
Tilia americana var. mexicana (Tilia) is a medicinal plant used in traditional medicine to control seizures. Different experimental models have suggested that organic extracts have anticonvulsant, neuroprotective, and hepatoprotective effects and antioxidant and cytotoxic activities (1-3). Although the anticonvulsant effects of Tilia were preliminarily explored on pentylenetetrazole (PTZ)-induced seizures in mice (1), Tilia organic extracts could act by abolishing the effects of PTZ as an antagonist of the GABAergic system; that is, Tilia organic extracts facilitate the inhibitory response mediated by GABA (1). There is no evidence of the anticonvulsant spectrum of Tilia activity in other models inducing seizures by increasing the excitatory response, such as kainic acid. Kainic acid (KA) induces seizures by acting as an agonist of the kainate subtypes of ionotropic glutamate receptors. It is used to induce focal seizures with complex symptomatology and secondary generalization from the limbic focus, due to epileptogenesis process (4). Limited information is available about the role of KA in systemic injury. This compound has been shown to induce oxidative damage in the brain, liver, and kidneys in experimental models (5, 6).
Additionally, another glutamate analog structurally similar to KA, domoic acid, has been shown to cause kidney damage (7). Very few studies have been performed on the central and peripheral protective effects of plants and plant extracts (8).
2. Objectives
This study aimed to determine the biological effects of Tilia organic extracts on rats’ behavior and biochemical parameter (lipoperoxidation) in KA-induced seizure model (an experimental model where the glutamatergic system mediates excitatory response and induces oxidative stress) in different brain regions, the liver, and kidneys.
3. Methods
3.1. Extract Preparation and Chemical Constituent Analysis
Tilia americana L. var. mexicana (Schltdl.) Hardin (Tiliaceae) was collected in Puebla, Mexico (2010). The botanical expert Susana Valencia-Avalos identified the species. A voucher specimen of the plant (number 131613) was deposited in the Herbarium of the Faculty of Sciences, National Autonomous University of Mexico, for future reference. To prepare the extracts, we dried leaves, crushed (15 g), and macerated them at room temperature (22°C) with solvents of increasing polarity. Preliminary degreasing of vegetal material was performed using hexane (1 L x 3 consecutive times). After filtration, the residue was macerated with EAc (1 L x 3 consecutive times), followed by ME (1 L x 3 consecutive times). Solvents were separated by gravity filtration and evaporated under vacuum to obtain the dry weight percentage (%DW), as follows: 3.01 g (1.4%) of a dark green semisolid EAc extract and 29.8 g (9.4%) of a brown powder ME extract.
An Acquity UHPLC-H class (UHPLC, Acquity Waters, Singapore) was used for chromatographic analysis. It was equipped with a Symmetry C18 column (100 Å, 150 mm x 4.6 mm, 5 mm, Waters, Ireland) at the thermostat temperature of 43°C. Acidified water with 0.1% phosphoric acid in Milli-Q water (A) and methanol (B) (HPLC grade) were used as the mobile phase. The initial gradient elution was set at 80% A:20% B to reach 100% B (17 min). The initial condition was regenerated for 3 min (80% A: 20% B). The flow rate was fixed at 1.0 mL/min with an elution curve of 6. The extract (3 mg/mL) was diluted in methanol/Milli-Q water (50:50) and injected directly after filtration through a 0.2 mm filter (GHP, acrodisc-13, Waters) in a volume of 10 µL. Commercial flavonoid standards (rutin, quercetin, isoquercitrin, quercitrin, and kaempferitrin; 2 mg/mL) were used as references.
3.2. Animals
Male Wistar rats (180 - 220 g, Mexico) were used in this study. Animals were housed under constant temperature (20 - 25°C) and humidity (50 - 60%) with 12/12-hour light/dark cycles. They were fed a standard commercial rat diet (Harlan Teklad Global diet 2018S sterilized, USA). All experimental procedures were performed according to guidelines and were approved as part of project 04-2013.
3.3. KA-Induced Status Epilepticus
Status epilepticus was induced with KA (10 mg/kg, i.p.). Changes in convulsive behavior were monitored over six hours, beginning immediately after the last treatment administration, according to the phases of crises previously reported (9) and considering Racine’s Scale (10): phase 0, without reaction; phase 1, stereotypies and/or mild facial clonuses and/or eye blinking; phase 2, head nodding and/or multiple facial clonus and/or the changes described in phase 1; phase 3, unilateral forelimb myoclonus and/or the changes described in phases 1 and 2; phase 4, bilateral forelimb clonus with rearing and/or the changes described in the previous phases; and phase 5, bilateral forelimb and/or hindlimb clonus (generalized clonic convulsions) with falling (loss of balance). Status epilepticus was established when behavioral seizures appeared at less than 3 min intervals for at least 5 min.
3.4. Experimental Groups and Tissue Preparation
The groups (n = 6) used in this study were as follows: (1) control (C), no substance administration; (2) phosphate buffer (PB; ME extract vehicle, 2 mL, p.o. using a neonatal/infant oral feeding tube); (3) olive oil (OO; EAc extract vehicle, 2 mL, p.o.); (4) ME extract (ME; 100 mg/kg, p.o.); (5) EAc extract (EAc; 100 mg/kg, p.o.); and (6) KA (10 mg/kg, i.p.). Groups 7 to 10 received KA and further treatment as follows: (7) PB + KA; (8) OO + KA; (9) ME + KA, and (10) EAc + KA. In particular, the Tilia groups received treatment three days before KA administration to assess protective effects. After four hours of behavioral observation, the animals were sacrificed, and the tissues were removed, homogenized, and centrifuged. The supernatant was used to determine lipoperoxidation levels.
3.5. Lipoperoxidation Determination
Lipid peroxidation was determined using the colorimetric thiobarbituric acid reactive substances (TBARS) method. The experimental procedure was performed as previously described (3).
3.6. Statistical Analysis and Data Interpretation
All data are presented as the mean ± standard error (n = 6). The effects of Tilia extracts on seizures and lipid peroxidation were analyzed using one-way ANOVA, followed by a post hoc Bonferroni multiple comparison test to determine differences between the groups. Differences with P < 0.05 were considered statistically significant.
4. Results and Discussion
4.1. Behavioral Assessments
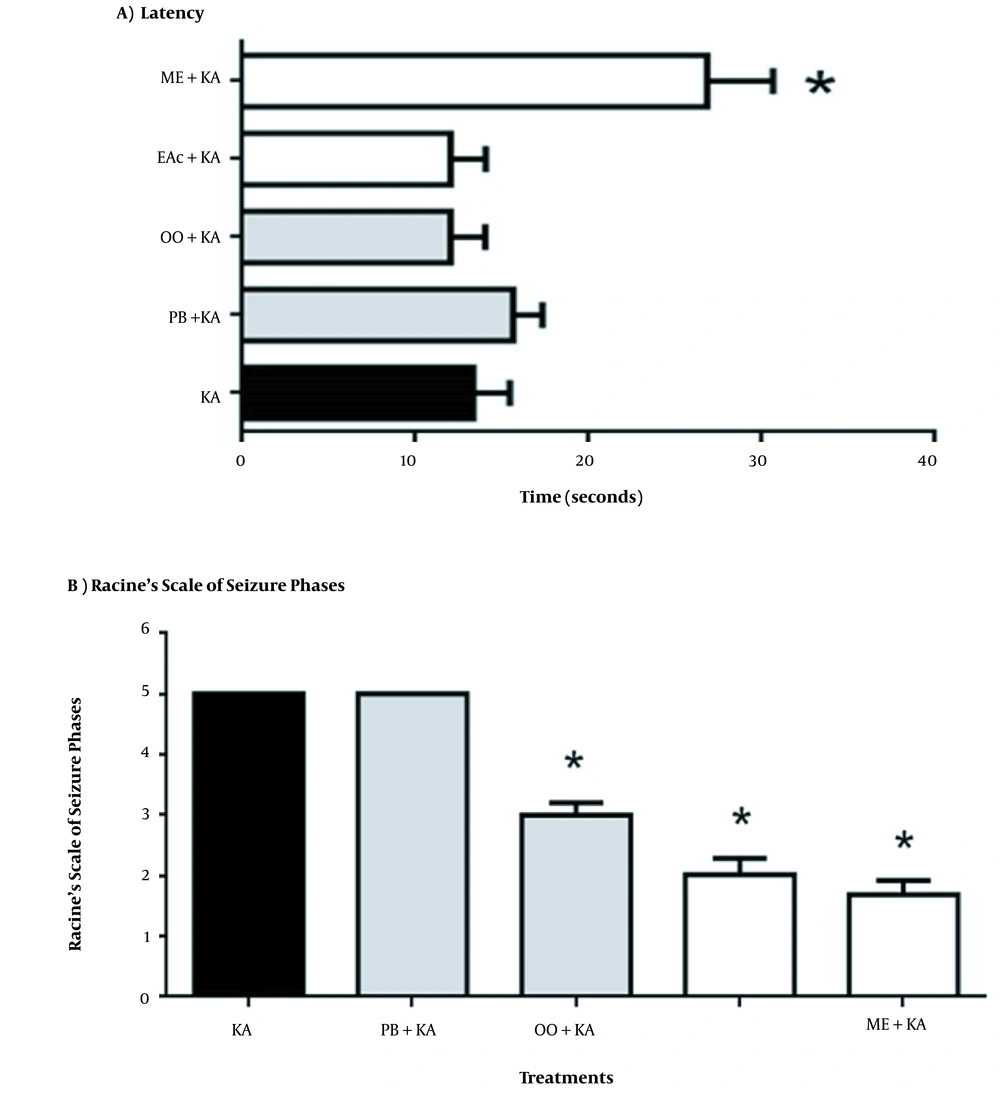
The latency to seizure onset (behavioral changes associated with phase 1 or 2) in the groups receiving treatment was not significantly different among the KA (13 ± 2.11 min), PB + KA (16 ± 1.6 min), OO + KA (12 ± 2 min), and EAc + KA groups (12 ± 2 min). However, a significant increase was observed in the ME + KA group (27 ± 1.6 min; P < 0.01; Figure 1A). The OO+KA group showed a decrease in the severity of the seizures, where we observed unilateral forelimb myoclonus and multiple facial clonuses (phase 3), while the groups treated with EAc and ME extracts showed behavioral changes associated with head nodding and/or multiple facial clonuses (phase 2) and eye blinking and/or facial clonuses (phase 1), respectively, during monitoring (Figure 1B).
(A) Latency to seizure onset in the groups receiving treatments with organic Tilia americana var. mexicana extracts or vehicle in the presence of KA. The data represent the mean ± SEM of n = 6 animals. ANOVA followed by Bonferroni multiple comparisons test. Abbreviations: Kainic acid (KA); plus olive oil (OO + KA); plus phosphate buffer (PB + KA); plus ethyl acetate extract of Tilia (EAc + KA); plus methanolic extract of Tilia (ME + KA). *P < 0.0001, ME + KA vs. all groups; (B) The effect of organic Tilia americana var. mexicana extracts or vehicle on limbic seizures induced by KA (Racine Scale). The data represent the mean ± SEM of n = 6 animals. ANOVA followed by Bonferroni multiple comparisons test. Abbreviations: Kainic acid (KA); plus olive oil (OO + KA); plus phosphate buffer (PB + KA); plus ethyl acetate extract of Tilia (EAc + KA); plus methanolic extract of Tilia (ME + KA). *P < 0.0001, ME + KA vs. OO + KA, vs. EAc + KA and vs. ME + KA.
4.2. Lipoperoxidation Data from Different Tissues
We also verified that KA administration increased the TBARS concentration (expressed as MDA) in the cerebellum (12.11%), brain hemispheres (14.92%), cortex (11.72%), medulla (10.96%) (Table 1), liver (24.36%), and kidney (25.25%) compared to the control (Table 2). The administration of ME and EAc Tilia extracts reversed the KA-induced increase in lipid peroxidation in the cerebellum (43.49% and 46.09%, respectively), brain hemispheres (38.76% and 50.10%, respectively), cortex (39.22% and 42.35%, respectively), medulla (42.17% and 46.18%, respectively), liver (68.68% and 65.94%, respectively), and kidney (67.58% and 76.73%, respectively). Moreover, we observed that the vehicle OO also acted against KA, decreasing TBARS levels by 37.22%, 38.83%, 59.35%, and 70.02% in the cerebellum, brain hemispheres, cortex, and medulla, respectively, and 59.82% and 50.76% in the liver and kidney, respectively, compared to the control group (all P < 0.05; Tables 1 and 2).
| Tissue | Dose Extracts/PB (mg/kg) | MDA (nM/mg/Protein) | |
|---|---|---|---|
| Without KA | With KA (10 mg/kg) | ||
| Cerebellum | |||
| C | - | 21.9 ± 3.3 | 180.8 ± 1.4* |
| OO | 1.5 mL | 23.1 ± 1.7 | 67.31 ± 2.0* |
| PB | 100 mg/kg | 27.3 ± 2.3 | 171.9 ± 2.5 ° |
| EAc | 1.5 mL | 37.7 ± 1.3 | 83.34 ± 2.1* |
| ME | 1.5 mL | 35.7 ± 2.1 | 78.68 ± 3.1* |
| Brain hemispheres | |||
| C | - | 20.02 ± 3.2 | 134.1 ± 6.0*, ° |
| OO | 1.5 mL | 24.73 ± 1.5 | 52.08 ±2.0* |
| PB | 100 mg/kg | 27.23 ± 2.6 | 126.2 ± 3.2* |
| EAc | 1.5 mL | 37.32 ± 1.7 | 76.3 ± 2.2* |
| ME | 1.5 mL | 33.1 ± 2.1 | 67.20 ± 5.1* |
| Cortex | |||
| C | - | 19.72 ± 3.5 | 81.2 ± 1.0* |
| OO | 1.5 mL | 20.61 ± 2.5 | 48.2 ± 2.8* |
| PB | 100 mg/kg | 28.35 ± 1.0 | 71.0 ± 2.9* |
| EAc | 1.5 mL | 35.0 ± 2.09 | 65.8 ± 1.7* |
| ME | 1.5 mL | 29.1 ± 2.3 | 67.2 ± 3.2* |
| Medulla | |||
| C | - | 11.30 ± 2.1 | 103.1 ± 1.3* |
| OO | 1.5 mL | 22.66 ± 2.1 | 72.20 ± 1.1* |
| PB | 100 mg/kg | 20.81 ± 1.2 | 96.30 ± 1.9* |
| EAc | 1.5 mL | 25.31 ± 2.2 | 47.61 ± 1.9*, ns |
| ME | 1.5 mL | 24.16 ± 2.2 | 43.57 ± 4.7* |
| Tissue | Dose/Volume Extracts/PB (mg/kg) | MDA (nM/mg/Protein) | |
|---|---|---|---|
| Without KA | With KA (10 mg/kg) | ||
| Liver | |||
| C | - | 76.1 ± 11.1 | 315.6 ±3.6* |
| OO | 1.5 mL | 97.3 ± 13.3 | 188.8 ± 3.6* |
| PB | 100 mg/kg | 102.4 ± 6.3 | 300.73 ± 0.5* |
| EAc | 1.5 mL | 147.7 ± 11.9 | 208.1 ± 8.0*, ns |
| ME | 1.5 mL | 126.4 ± 8.7 | 206.7 ± 3.11* |
| Kidney | |||
| C | - | 71.1 ± 3.3 | 281.5 ± 27.5* |
| OO | 1.5 mL | 71.2 ± 6.8 | 142.9 ± 44* |
| PB | 100 mg/kg | 85.5 ± 3.4 | 250.7 ± 46.3* |
| EAc | 1.5 mL | 147.5 ± 1.9 | 216.5 ± 4.3* |
| ME | 1.5 mL | 137.3 ± 6.4 | 190.3 ± 23.2* |
We assessed the effect of Tilia americana var. mexicana leaf extracts on MDA (nM/mg/protein) in the brain after KA-induced injury. The effect in the KA group was compared to that in all the other groups: cerebellum: F (9,50) = 4511.0, *P < 0.0001 vs. all groups, °P < 0.001 vs. PB; brain hemispheres: F (9,50) = 632.6 *P < 0.0001 vs. all groups, °P < 0.001 vs. C; cortex: F (9,50) = 371.7, *P < 0.0001 vs. all groups; and medulla: F (9,50) = 1239.0, *P < 0.0001 vs. all groups, not significant (ns) for EAc + KA vs. ME + KA. The data represent the mean ± SEM of n = 6 animals (in triplicate samples). The ANOVA was followed by Bonferroni multiple comparison test (Table 1).
The effect of Tilia americana var. mexicana leaf extracts was also assessed on MDA (nM/mg/protein) in the liver and kidney after KA-induced injury. The effect in the KA group was compared with that in all the other groups: liver: F (2.53,12.66) = 17.81, *P < 0.0001 vs. all groups, not significant (ns) for EAc + KA vs. ME + KA; kidney: F (9,50) = 2389, *P < 0.0001 vs. all groups. The data represent the mean ± SEM of n = 6 animals (in triplicate samples). The ANOVA was followed by Bonferroni multiple comparison test (Table 2).
4.3. Chromatographic Profile of Tilia Leaf Methanol (ME) and Ethyl Acetate (Eac) Extracts
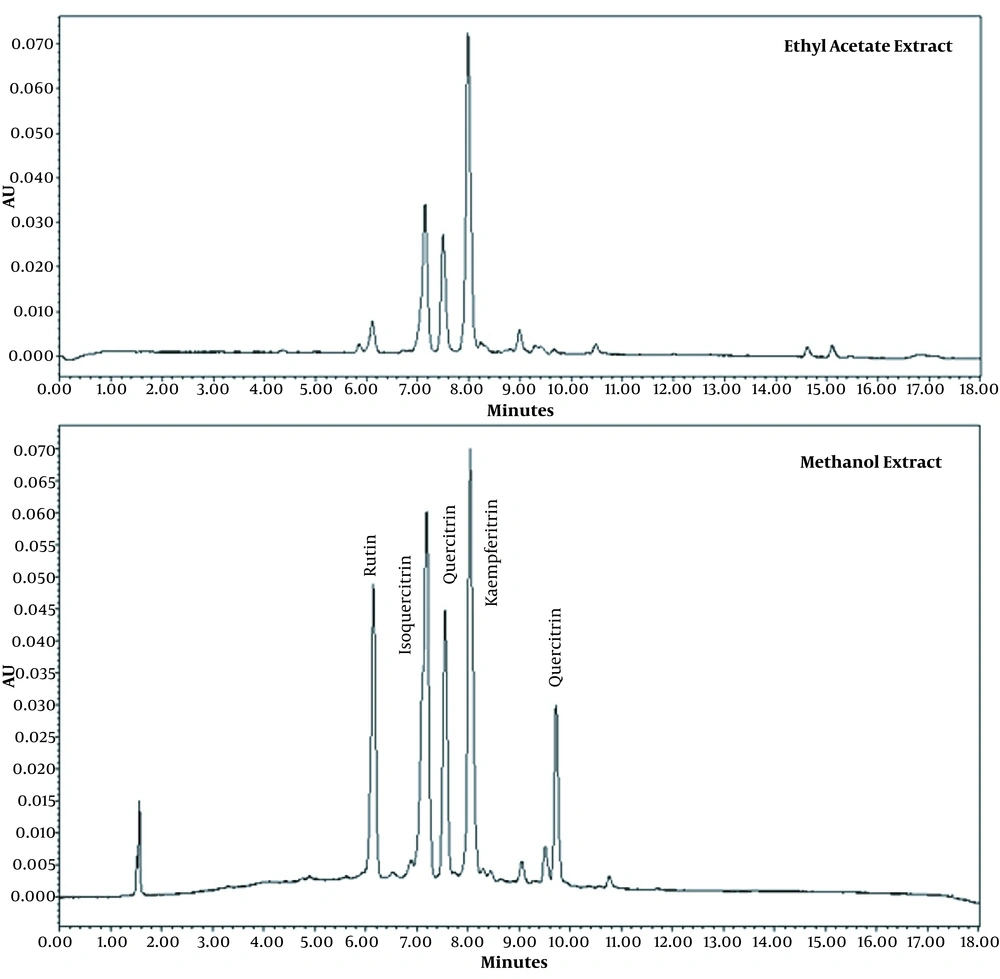
The environment is an essential factor that might significantly affect the biochemical constituents of a plant extract. The chromatographic analysis of Tilia extracts obtained from different regions of Mexico demonstrated that quercetin and kaempferol derivatives were the main bioactive constituents and marker compounds for guaranteeing the standardization of extracts (11-13). The presence of glycosides derived from quercetin (rutin, isoquercitrin, and quercitrin) and kaempferol (kaempferitrin) flavonoids was confirmed in the chromatographic profiles of both leaf extracts (Figure 2).
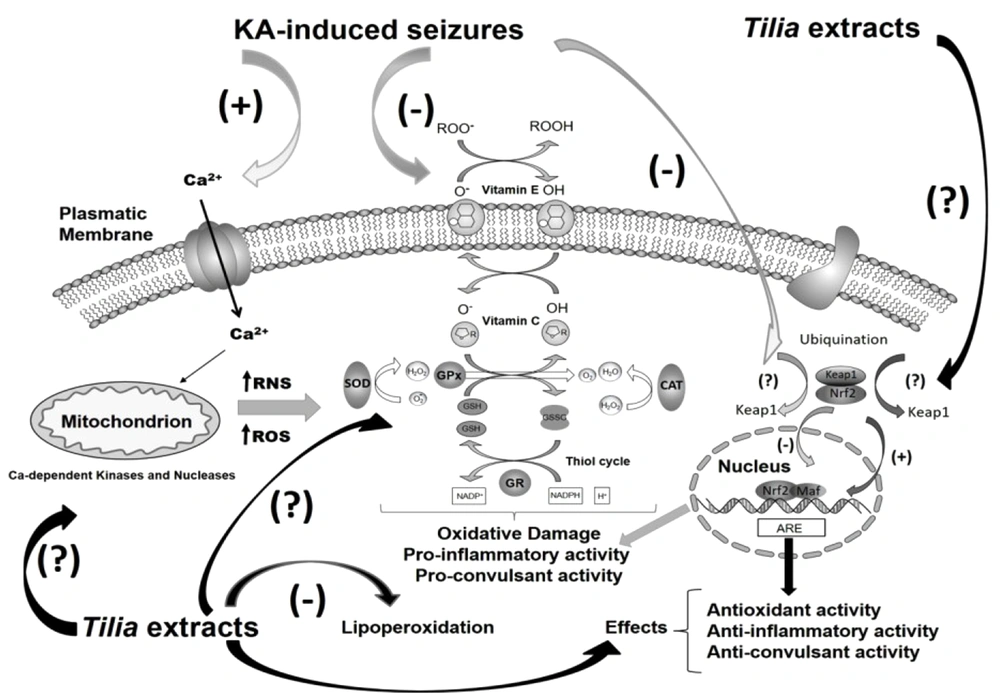
In this study, we showed, for the first time, that the levels of lipid peroxidation in the liver and kidney were significantly reduced in the groups treated with EAc and ME extracts and KA compared to those treated with KA alone. Our results also showed that the vehicle OO effectively diminished the lipoperoxidation levels in all tissues. These effects can be mainly attributed to quercetin, quercetin glycosides, and kaempferol in Tilia extracts. The antioxidant effects of the vehicle OO can be attributed to the phenol content and monosaturated fats. It is known that OO has different hydrophilic and lipophilic phenols (14). The main phenolic compounds in OO are phenolic alcohols, phenolic acids, flavonoids, lignans, and secoiridoids, with the class of secoiridoids (oleuropein, ligstroside, and oleocanthal) being the most representative in OO showing strong antioxidant properties (15, 16). These results suggest that systemic damage alters the central nervous system (CNS) activity, but Tilia and OO can abolish these changes. A few studies that evaluated potential KA-induced damage at the systemic level and its impact on the CNS found an increase in oxidative stress markers in the brain, liver, and kidney (5, 6, 8). The presence of glycosides derived from quercetin and the aglycone quercetin, as well as kaempferitrin, was confirmed in the ME extract of Tilia leaves. Some of these flavonoids were also detected in the studied extracts. These metabolites have been obtained from the inflorescences or leaves of this and other Tilia species (13, 17), and biological effects on the CNS have been reported (1, 3, 17-20). Concerning OO, phenolic compounds can counteract oxidative stress in brain tissue (21). In particular, oleuropein and hydroxytyrosol act as direct free radical scavengers, hydroxytyrosol and oleocanthal are cyclooxygenase inhibitors, and oleuropein acts against lipoperoxidation (15, 22-24). The anticonvulsant and antioxidant properties of Tilia species, as well as its abundant flavonoids, could be associated with the ability of ME extract to protect against KA-induced oxidative damage at the central and systemic levels. Olive oil could be protective at the central and systemic levels due to its anti-inflammatory and immunomodulatory properties induced by the presence of phenolic compounds and monounsaturated fats, as these compounds affect energy metabolism (25). Hydroxytyrosol, tyrosol, and oleuropein compounds have also been shown to be protective through Nrf2 activation, a pathway involved in the synthesis of antioxidant enzymes (16, 24, 26, 27). We postulate that antioxidant agents are beneficial for mitigating neurotoxicity and CNS damage induced by glutamate activation. As shown in our lipid peroxidation results, OO and the ME and EAc extract further reduced the KA-induced damage in different brain areas involved in the propagation of neuronal damage (the limbic system: hippocampus, amygdala, and piriform cortex) associated with the oxidative stress induced by KA administration (9, 28, 29). The administration of Tilia extracts or vehicle OO before KA injection had neuroprotective effects, probably involving antioxidant activity that prevented the spread of systemic damage to other tissues. Based on the last experiments in this study, we hypothesize that Tilia extracts have antioxidant activity that modulates ROS production and thereby decreases oxidative damage by other mechanisms in the brain (Figure 3). In terms of OO, it has been shown in a pentylenetetrazole-induced mouse model of epilepsy that oleuropein has anticonvulsant properties through anti-inflammatory, opioidergic, and nitrergic pathways (30, 31). Oleuropein has also been shown to have neuroprotective effects in a KA rat model of epilepsy, reducing MDA, nitrite, and nitrate levels and increasing GSH levels in addition to showing antiapoptotic effects (32). The anti-apoptotic, anti-inflammatory, and antioxidant effects of OO in the epileptic brain also show benefits in peripheral tissues (32). It is important to notice that since the solvent of ME extract was phosphate buffer, the results reflect the favorable effects of this plant extract. In the case of EAc extract, the solvent was olive oil, which had favorable effects on the criteria per se. Nevertheless, it did not create a synergistic effect with the EAc extract. Therefore, it is suggested that ME extract be considered in future studies to evaluate the net effects of this plant. Consequently, according to the favorable effects of both olive oil and the EAc extract, their combined effects should be further evaluated. Finally, the systemic effects could involve another biochemical mechanism that will be investigated in the future.
Mechanisms proposed for the antioxidant and anti-seizure effects of the organic extracts of Tilia americana var. mexicana in the brain. Reactive oxygen species (ROS) production is accompanied by the activation of enzymes involved in ROS scavengings, such as superoxide dismutase (SOD), catalase (CAT), ascorbate-glutathione cycle enzymes (GR, GPx), and glutathione reductase (GR). KA: Kainic acid.
4.4. Conclusions
Kainic acid damages the CNS and the periphery, as evidenced by increased lipid peroxidation levels in the brain, kidney, and liver. Tilia extracts and the vehicle olive oil decrease KA-induced seizure severity and lipid peroxidation in the brain and other organs explored, suggesting that their bioactive constituents participate through antioxidant properties. The vehicle olive oil possibly shows anticonvulsant activity through the antioxidant properties mediated in part by its main reported active component, the secoiridoid oleuropein. Regarding the extracts and their vehicles, it will be interesting to explore in the future in more detail the net effects of the plant associated with the constituents of Tilia ME extract, while the favorable effects obtained in the combination of olive oil and EAc extract can also be further evaluated.