1. Background
Health budget constraints, increasing new expensive life-saving drugs, and enhanced public health expectations have increasingly required manufacturers to prove the value of their drugs for payers and budget holders. However, the available evidence at the time of drug registration is often insufficient to accurately estimate the clinical effectiveness, cost-effectiveness, or budgetary impact of a drug in the real world (1-3). Although the efficacy of drugs is approved through clinical trials, their effectiveness and utilization in the real world are uncertain before marketing (4). This uncertainty may lead to delayed decision-making in terms of access and reimbursement (5). Delay in reimbursement and the risk of rejection for inclusion in the positive list may discourage the industries from investing in new innovative medicines with high risk and low market potential, like orphan drugs or personalized medicines (6, 7).
Several policies are implemented worldwide to manage and confine such uncertainties (8). The best solution for passing this hurdle is a formal institutional agreement between pharmaceutical companies and payers to share the associated risks deriving from the administration of innovative pharmaceutical technologies (9). This approach varies from traditional reimbursement methods in which healthcare payers accept almost all risks (10). Various names are used to introduce and describe this general plan, such as risk sharing agreement (RSA), Performance-based risk sharing agreement (PBRSA), and patient access scheme (PAS), all of which are recently summarized with the concept of managed entry agreement (MEA), the name we adopted in this article (2). These contracts are applied for various purposes in different countries; however, the main benefits of these contracts include accelerated access to new drugs, fast reimbursement of new drugs, real-world evidence generation, and dealing with uncertainties (9).
Although all of these agreements aim to accelerate patients' access by sharing the risks, there are significant contrasts between their main categories, such as "financial-based," "outcome-based," and "coverage with evidence development" (1). The arrangements falling within the first category may include price-volume agreements, discounts, and dose/time capping schemes. Controlling and managing budget impact based on financial metrics (e.g., total sales) or real-world utilization are the main objectives of these agreements. For example, in price-volume agreements, the price for each drug unit is determined in light of its sales. On the contrary, outcome-based agreements mainly include outcome guarantee, money-back guarantee, conditional treatment continuation, and process of care as the main types in this category that usually address uncertainty regarding the clinical and/or cost-effectiveness of new medicines. In addition, they play a significant role in managing budget impact and utilization.
Various mechanisms are used in different methods for achieving the above-mentioned aims. For instance, in outcome-guarantee agreements, payment is made only by patients who respond to treatments, and if a product fails to achieve an agreed-upon clinical result, the producer offers or agrees with rebates, refunds, or price changes if applicable. Positive coverage decision in the "coverage with evidence development" agreements is based on the collection of additional evidence (only with research or only in research), which might result in continuing, extended, or discontinued coverage (1, 11, 12).
The growing application of such agreements in recent years is a response to the high price of new medicines (especially anti-cancer and orphan drugs), limited budget of health system payers despite rising costs, uncertainty around the clinical effectiveness of drugs in the real world, attention to patients' unmet medical needs, and acceleration and improvement of patients' access to new drugs (13, 14). Although high-income countries implementing such agreements have considerable experience, limited experience is available in developing countries like Iran. The MEA has recently comprised one of the principal plans pursued to facilitate European patients' access (15). In contrast, developing countries are less experienced in implementing such arrangements (16). Ferrario et al. (17) examined the implementation of MEAs in the Central and Eastern European (CEE) countries, reporting that the most significant number of MEAs were implemented in Estonia, Slovenia, Hungary, Latvia, and Romania.
Conversely, Slovakia, Russia, Kosovo, and Albania did not have any MEA records when the study was conducted. Among the countries that did not have experience in implementing MEAs, Slovakia has taken a step towards implementing these agreements by changing the pricing and reimbursement legislation. However, based on federal law in Russia, all public purchases must be made through tenders, with the lowest price determining the winner, so there is no experience in this area. Although the private sectors in Russia have a different position, in theory, they deem MEAs too complicated for their routine needs and do not approach these agreements (17).
Maskineh and Nasser (18) explored the experience of the Middle Eastern and North African (MENA) regions in implementing MEAs for medical products. The results showed that only a few countries employed MEAs, which may be attributed to the lack of data collection infrastructure and insufficient expertise in health economics. At the same time, the study indicated that healthcare stakeholders had a positive attitude toward the potential of MEAs and projected an increase in their implementation to address budget impact while improving access to innovative drugs (18). However, previously published articles did not consider MEA in Iran as a developing country in the MENA region.
Ansaripour et al. describes a systematic process of assessing, appraisal, and judging drug reimbursements in Iran that were overseen by the two most influential bodies, including the Iran Food and Drug Administration (IFDA) and the Supreme Council of Health Insurance (SCoHI) (19). The organizations that offer health insurance in Iran are subsumed under three groups based on their functional nature. The first group, known as Social Health Insurance, covers about 90% of Iran's population and includes three main insurance funds – namely the Iran Health Insurance Organization (IHIO), the Social Security Organization (SSO), and the Armed Forces Medical Services Insurance Organization (AFHIO). These organizations generally cover 70% and 90% of the costs of outpatient and inpatient services, respectively. The second group, known as Institutional Health Insurance Funds, involves organizations like the Petroleum Industry Health Organization, the National Broadcasting Organization, and banks. Finally, the third group entails Commercial Health Insurance Organizations that work voluntarily and offer private supplemental insurance (20). The process of reimbursement decision-making in Iran is associated with challenges, including budget constraints of the Iranian Insurance System and managing the recommendations of various stakeholders with different interests, leading to conflicts of interest and delays in reimbursement decisions, which, in turn, reduces patients' access to innovative drugs and increases out-of-pocket payments. These challenges highlight the necessity of applying new reimbursement policies (19).
Although the Iranian SCoHI has adopted new policies in 2020, there is no formal experience in implementing MEAs in the field of pharmaceuticals and medical devices in Iran. This has caused patients' reduced access to novel and costly drugs. The new policies incorporate some models of financial-based agreements (e.g., price-volume agreements and patient utilization cap), numbers of outcome-based agreements (e.g., outcome guarantee, money-back guarantee, and patient registry), and both models of coverage with evidence development (only with research or only in research). Before developing these new policies, the reference base pricing (RBP) and strategic purchasing were the main reimbursement policies applied in Iranian insurance organizations (21).
Since the implementation of MEAs can affect the benefits of different stakeholders, seeking the insights of stakeholders can help in the optimal implementation of a new policy in the Iranian reimbursement system. The current study explored the principal stakeholders' opinions, including payers, manufacturers, and patients representatives. Our particular interest was to assess stakeholders' views on the objectives, pros, and cons of implementing MEAs in Iranian health insurance. The findings will hopefully help decision-makers enhance the process of implementing MEAs and improve their insights into the best pathway to pursue these policies.
2. Methods
Based on the literature, the goal of MEAs implementation is to respond to uncertainties related to the launch of new and expensive drugs (13). Despite existing similarities in different contexts, uncertainty cases vary among different countries regarding importance and priority (9).
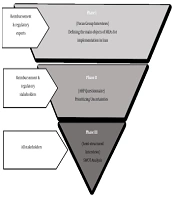
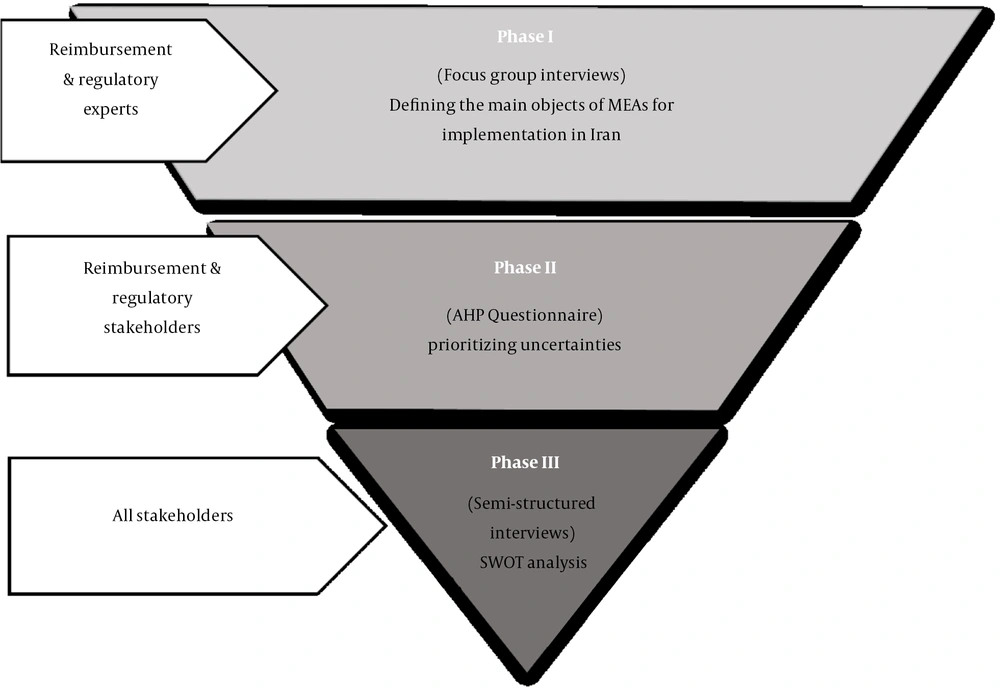
Overall, the research methods adopted in this study contained three phases (Figure 1): (1) Conducting focus group interviews (FGIs) with insurance and regulatory experts to reveal the main objectives of implementing MEAs in Iran, (2) developing a questionnaire through adopting an analytical hierarchy process (AHP) approach to prioritize uncertainties, and (3) running individual semi-structured interviews with all stakeholders involved to carry out strengths, weaknesses, opportunities, and threats (SWOT) analysis.
To answer the first research question (i.e., identifying the main objectives of MEA implementation and exploring the main uncertainties faced by third-party payers in Iran), we conducted two FGIs using purposive sampling. The first FGI was held at the IFDA with subject matter experts from third-party payers and IFDA regulators. Seven experts participated in the first session. The second FGI was held at the SCoHI, with nine members of the Committee for Review and Development of Drug Commitments. This committee included representatives from the health insurance organizations, the Ministry of Health and Medical Education, the IFDA, and the SCoHI. Before the FGIs, a set of questions were developed in the form of an interview guide to investigate the objective of MEAs and identify critical uncertainties. To guide the focus group, we considered one moderator and one supervising professor who encouraged the participants to brainstorm to generate an initial list of uncertainties. Following this method, the moderator wrote all of the opinions on the board and omitted or confirmed them after consensus. After criticism and consensus, the identified uncertainties were classified into different categories according to their relationship. Additionally, interviewees were free to express additional perspectives on the topic, allowing us to understand the participants better. The recorded focus groups were subsequently transcribed and subject to thematic analysis using MAXQDA to detect major uncertainties.
The main objectives and uncertainties from the FGIs were grouped into themes and used to evaluate and prioritize through an AHP approach. For this purpose, an AHP questionnaire was designed and completed by 16 experts who had participated in the FGIs. Except for two questionnaires excluded due to missing data, other questionnaires were analyzed using Expert Choice 11 software.
For the second research question, we sought to explore stakeholders' insights into the SWOT of MEAs and elicit their suggestions for the optimal implementation of such agreements. To this end, we conducted 17 in-depth semi-structured interviews with all stakeholders involved, including five insurance representatives, two regulatory representatives, six industry representatives (providers encompassing both manufacturers and importers), and four patient representatives (including physicians and patients) using an interview guide. Designing the interview guides for both the focus groups and individual interviews was based on Ferrario and Kanavos (3) and Garrison et al. (12). Six interviews were conducted on the phone, while the rest was done in face-to-face meetings. All interviews were recorded and transcribed verbatim. We continued the interviews upon reaching theoretical data saturation (i.e., a point where the interviewees' already identified themes were repeated and no new piece of information was divulged). The transcriptions were subject to thematic analysis using MAXQDA Analytics Pro 2020. We received verbal informed consent from all interviewees for collecting and using personal information and audiotapes of interviews or FGIs. Data collection in three phases lasted from August 2019 to March 2020.
3. Results
3.1. Screening of Uncertainties in Iran from Stakeholders' Perspectives
The first and second main objectives of MEAs were to improve patients' access to innovative and expensive drugs and respond to uncertainties around reimbursement of new medicines, respectively. The uncertainties emerging from the FGIs in our study entailed a wide array of subjects, which were classified under three following categories: (1) Uncertainty around budget impact caused by introducing new drugs into the reimbursement list: Drug price and patient population were the main factors in this domain. (2) Uncertainty regarding cost-effectiveness: This category included factors like clinical effectiveness, safety, how the drug affects patients' quality of life in the real world, and the absence of therapeutic guidelines. (3) Uncertainty around the real-world utilization: This category consisted of factors such as uncertainty related to off-label use, patient adherence, and physicians' prescription pattern.
The most recommended groups of medicines for MEAs by FGIs participants were as follows: (1) High-tech and expensive medicines and/or medicines with a high cost of treatment; (2) drugs with the possibility of smuggling, abuse, or induced demand; and (3) drugs with a limited market (e.g., orphan drugs).
In addition, our interviewees believed that MEAs durations should be different depending on the type of drug and agreement. They suggested that the suitable duration for outcome-based agreements depends on their required time to catch the outcome, with the optimal duration of five years. On the contrary, the optimal duration of financial-based agreements should be shorter, ranging from two to three years.
3.2. Prioritization of Uncertainties
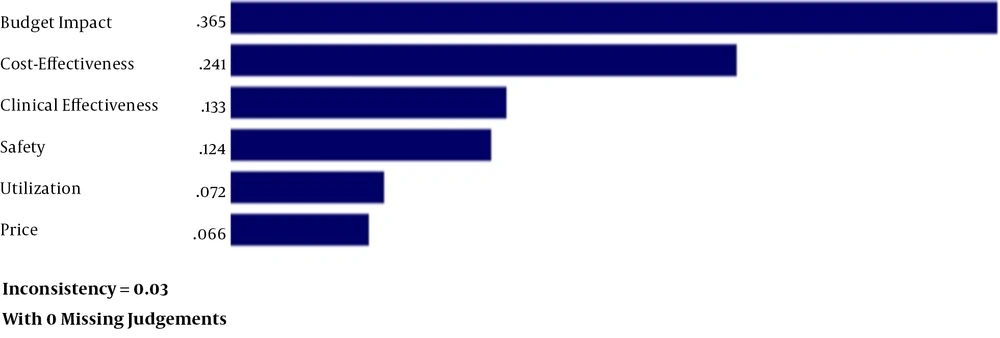
To prioritize uncertainties, we performed a pairwise comparison using the AHP approach. Overall, the data obtained from the 14 completed questionnaires were entered into Expert Choice, a multicriteria decision-making software program. The AHP is a structured technique for organizing and evaluating the importance of different criteria to select the best alternatives.
As shown in Figure 2, uncertainty about budget impact had the highest priority, followed by cost-effectiveness, clinical effectiveness, safety, real-world utilization, and unit price of the drug.
3.3. Strengths, Weaknesses, Opportunities, and Threats
Conducting 17 individual semi-structured interviews yielded around 250 minutes of recording, which were subsequently transcribed and analyzed. We assigned the preliminary codes to our data using the thematic analysis approach and then searched for patterns or themes in the codes. Relevant and recurrent themes describing the advantages and disadvantages of MEAs and facilitators for implementing these agreements in the reimbursement system are discussed below. The initial 76 identified codes were subsumed under 24 latent codes and finally illustrated as a SWOT table (Table 1).
| SWOT Analysis | |
|---|---|
| Strengths of MEAs | Weaknesses of MEAs |
| From providers' perspective: Creating a monopoly during the term of the agreement; Obtaining earlier access to the market; Creating competitive advantages for products subject to MEAs; Facilitating the drug reimbursement process; Maintaining assured market access during the term of the agreement | Lack of transparency in the implementation of MEAs; High transaction costs, including legal complexity, the time-consuming nature of MEAs, and the difficulty of obtaining an executive guarantee; Difficulty in defining, measuring, and evaluating meaningful/relevant outcomes in some disease areas (in the case of outcome-based MEAs) |
| From payers' perspective: Ensuring easier monitoring of market trends; Ensuring systematic thinking and clear decision-making in drug reimbursement; Increasing flexibility of drug coverage due to the wide variety of reimbursement procedures; Holding suppliers accountable for their commitments; Allocating resources more efficiently | |
| From patients' perspective: Accelerating access to medications; Receiving a broader range of high-quality services; Feeling satisfied; Ensuring health outcomes | |
| Opportunities of MEAs | Threats of MEAs |
| Reviewing drug packages with greater transparency and openness; Improving the efficiency of post-market studies through data collection requirements under MEAs | Provider's reluctance to engage (provider push-back); Lack of appropriate infrastructure to implement MEAs; Lack of patient cooperation in conducting studies and collecting their data; Responsibilities for data collection and associated costs; Lack of integration and coordination in the decision-making process |
Cross-coding was performed to ensure inter-rater reliability. More precisely, the first and second authors initially coded the data. After independent coding by two authors, if any disagreements occurred, the research members first tried to convince each other, and if they did not reach a consensus, the third person from the research team would judge the identified codes. Given the dynamic nature of the coding process and qualitative data analysis, the identified themes were reiteratively modified throughout the data analysis process.
One of the challenges mentioned by the stakeholders as a threat to the implementation of such MEAs in Iran is "the absence of proper infrastructure to implement MEAs." In this regard, the interviewees believed that the main challenges are the lack of information technology (IT) infrastructure to record and track patients' data and legal infrastructures. Concerning the absence of proper IT infrastructure, stakeholders believe that "design an integrated and traceable information recording system" is crucial for MEAs implementation. In this respect, one of the foreign company's representatives in the private sector stated, "We do not have proper access to the internet even in big cities, let alone villages and deprived areas." Also, a manager of a local producer company in the public sector said, "The electronic prescription has only been implemented in some provinces and cities, and there have been shortcomings in its implementation." Nonetheless, the interviewed payers claimed that "Iran has the required infrastructure to implement MEAs properly."
Considering the absence of legal infrastructure to guarantee MEAs, the interviewed stakeholders proposed that "expert lawyers should be consulted throughout the process of negotiating and signing MEAs" and "MEAs should be written in a transparent and binding manner so that it creates an obligation for all parties involved in the agreement." In this regard, the representative of the SSO said, "We need to equip ourselves by recruiting legal advisors for writing contracts." A suggestion proposed by the representative of the SCoHI to prevent legal problems was "establishing an intermediary organization for agreements with pharmaceutical firms." Our stakeholders pointed out that providers' resistance to engaging in MEAs, known as provider push-back (2), is a potential threat to these agreements. In their opinion, it happens due to the unpreparedness of the industry, on the one hand, and improper pricing, high discounts requested by insurers, and subsequently firms' reduced profit margins, on the other hand. The interviewees further offered some suggestions to help the industry get more prepared to enter into such contracts. These suggestions include "defining local and global Standard Operating Procedure (SOP) for implementing MEAs, establishing communication between pharmaceutical firms and physicians to control drug use, and developing patient support programs."
Another threat surrounding MEAs is the "lack of patients' cooperation in conducting studies and recording their data." In this regard, the representative of thalassemia patients said, "Experience has indicated that when a new drug is added to our patients' treatment basket, they say 'are we laboratory mice?' But if they are provided with enough information, they will cooperate." The representative of hemophilia patients also said, "The situation must be prepared [for patients' cooperation]. And a sense of camaraderie must be created between medical staff and patients. Incentives should also be offered."
According to the interviewees' views, data collection responsibilities and related costs are another threat to properly implementing MEAs, especially in outcome-based agreements. To address this problem, most interviewees suggested that "the costs should be covered by pharmaceutical firms, which ought also to be responsible for data collection or share the responsibility under the supervision of insurance companies." In this regard, one of the insurance representatives stated, "It is impossible to implement MEAs unless pharmaceutical firms share costs and cooperate in data collection and patient training." However, as one of the regulatory representatives asserted, "In collecting patients' data, pharmaceutical firms cannot be fully trusted and should not be left to themselves. As it is likely that firms manipulate data in favor of their drugs, payers must therefore supervise the process of data recording."
4. Discussion
Managed entry agreements have been used worldwide over the past 20 years as an effective management and cost containment tool to address uncertainties associated with the financial and clinical consequences of introducing innovative and expensive drugs (18). Australia, the European Union, the United States, and Canada have the most experience in implementing MEAs (22).
The current study provides rich qualitative evidence on the perspectives of Iranian stakeholders about MEAs implementation using two qualitative data collection procedures (i.e., focus group and individual semi-structured interviews). The study also explored the stakeholders' suggestions for optimal implementation of MEAs. Moreover, the identified uncertainties were quantitatively ranked and weighed by running an AHP approach. The primary purpose for payers to implement such agreements is to increase flexibility in improving patients' access to new and expensive drugs. Facilitating drug access is a significant aim shared by the entire health system (23). Another purpose for implementing MEAs mentioned by the stakeholders in the present research is responding to the uncertainties related to new drug entry. Uncertainties entail cases where no accurate estimation can be made because of insufficient evidence or information. As more information is obtained about a particular case, the degree of uncertainty declines (24). According to the findings, the principal uncertainty for decision-makers in the reimbursement system of Iran is the budget impact of drugs. Research suggests that responding to this uncertainty is the main reason for implementing MEAs in Belgium, the Czech Republic, France, Portugal, the United Kingdom (UK), and Lithuania. At the same time, managing cost-effectiveness and clinical effectiveness is a primary objective of implementing MEAs in countries like the Netherlands, Sweden, and Italy (3).
Given the concerns of payers in Iran, the best type of MEAs to respond to budget impact uncertainty is financial-based agreements. The main advantage of such agreements is that they can be easily implemented without the need for any complicated infrastructure. In parallel, a previous study has indicated that the most considerable models of MEAs implemented in the MENA region (71%) are financial-based (18). Some types of financial-based agreements that lead to cost-saving are discounts, cost/dose caps, price-volume agreements, and utilization caps (7). Among them, discount agreements are the easiest to implement, with negotiation skills being the primary tool in this type of agreement. However, the need for confidentiality often decreases payers' negotiation power. Compared to discount agreements, capping and price-volume agreements are more complicated to implement, in which it is necessary to access drug sales information and convince physicians to prescribe according to the guidelines (25).
Outcome-based agreements were designed to respond to the uncertainties around cost/clinical effectiveness and budget impact. Successful implementation of these agreements requires positive interaction between patients and physicians, transparent and measurable outcomes, patient registries, proper IT infrastructure, and the ability to negotiate and sign optimal agreements from a legal perspective (11, 25). Based on the threats mentioned by the interviewees in this study, outcome-based agreements in Iran will face some challenges like lack of appropriate IT and legal infrastructure and patients' unwillingness to cooperate in registering their information. Our stakeholders suggested that a comprehensive system be designed to record patients' information and monitor them to minimize the threats in these agreements. Besides, legal advisors must be consulted in all stages of writing and signing agreements, and an intermediary organization for agreements with pharmaceutical firms should be established. As a pioneer country, Italy has the largest number of implemented outcome-based agreements globally. Since 2000, intending to implement such agreements, this country has spent several million Euros to set up an electronic system for registering patients' information and electronic prescriptions (26, 27).
The implementation of MEAs is fraught with challenges, even in developed countries. Some of these challenges have to do with the internal weaknesses of such agreements, while others are related to external threats (28). One of the major challenges to properly implementing outcome-based agreements in the US and EU is selecting and measuring relevant outcomes (29, 30). Similarly, our interviewed stakeholders of the present research listed some of the outcome-based agreements' weaknesses blocking their proper implementation, including difficulty in definition, measurement, and evaluation of meaningful/relevant outcomes in some disease areas. Lack of transparency in the implemented agreements, based on European experiences, is also one of the main weaknesses of MEAs that resonates with our findings. This hinders interstate learning and limits patients' interaction with the MEA process (13). This pitfall originates from the failure to publish the details of MEAs implemented in a particular country in other countries due to confidentiality issues (10).
For years, policymakers in Iran pursued rigorous and inflexible methods (e.g., RBP) for drug pricing and reimbursement (31). The "high flexibility of MEAs resulting from the wide range of reimbursement methods" is one of the most frequently cited strengths of MEAs and an important reason why Iran has chosen MEAs as an innovative insurance policy. Besides all these strengths, two important opportunities were also mentioned by the participants in our study. "Reviewing covered drug packages with more transparency and openness" was the first opportunity mentioned by the stakeholders in the current study. In 2009, the UK decided to delist some Multiple Sclerosis medicines that had received insurance coverage for years but were not cost-effective, according to the National Institute for Health and Care Excellence (NICE). These medicines were then included in a 10-year outcome-based agreement, with the primary outcomes being published every two years. At the end of the 10-year period, while one of the drugs was not approved, the others obtained confirmation in the form of confidential discount agreements provided that they were subject to price cuts (32). Another opportunity mentioned by the interviewees in this study was that the MEAs enhance the efficiency of post-marketing surveillance because data collection is an integral part of such agreements. This is in line with the findings of previous studies (3).
The value of MEAs compared to traditional mechanisms depends on the characteristic of the product, disease, and the presence of required infrastructure for data collection and analysis (12). In South Korea, MEAs are implemented for pharmaceuticals with the following eligibility criteria: anticancer or orphan drugs with a lack of alternatives to treat severe and life-threatening conditions (33). In comparison, our stakeholders suggested that high-priced and low-market drugs are eligible for these agreements. They pointed out that drugs that could be smuggled or abused are also good candidates for MEAs. According to our stakeholders, the optimal duration of these agreements depends on the type of drug and agreement. Based on the type of agreements, it can vary from two years for financial-based to five years for outcome-based. The average duration of MEAs in the CEE countries is two years (ranging from one to five years). Moreover, discount agreements constitute the largest proportion of MEAs (73%) in this region, and the largest number of implemented agreements are registered for antineoplastic and immunomodulatory drugs (17).
Countries that apply MEAs as a tool for improving access to medicines should establish explicit objectives for their implementation. Our finding highlighted the main objectives of MEAs in the context of an emerging country in this area. Consuming the views of related stakeholders is essential for starting any new policy; otherwise, it will lead to policy failure.
Paying attention to the strengths, weaknesses, opportunities, and challenges of MEAs that threaten the implementation of these contracts helps policymakers design an efficient roadmap for the optimum administration of these agreements in Iran.
4.1. Study Strengths and Limitations
To the best of our knowledge, this study was the first systematic attempt to explore stakeholders' perspectives on implementing MEAs in Iran using multiple methods. Countries that have not yet experienced the application of such agreements may use this study as a first step toward the optimal implementation of MEAs. However, one of the study's limitations was the stakeholders' lack of experience in implementing MEAs. As a result, the respondents did not fully master all of the details and technical and operational infrastructure required by these agreements. In addition, the statistical population of eligible participants was minimal.
4.2. Conclusions
This multi-method study indicated that the current level of interest in MEAs beyond our main stakeholders is high, and they are optimistic regarding the potential of implementing these agreements in Iran. Ferrario and Kanavos indicated that despite similar uncertainties and reasons for implementing MEAs, countries generally adopt different MEA models for similar drugs (9). This discrepancy stems from existing differences in the health system and health-related executive policies in various countries. Therefore, decision-makers in Iran need to consider the peculiarities of the Iranian healthcare system when executing policies and selecting appropriate MEAs.
The analysis of the strengths, weaknesses, opportunities, and threats identified in this study will enable the key decision-makers of these agreements to gain more information about their details. Moreover, following the issues raised by the stakeholders, focusing on the required infrastructure to execute MEAs, facilitates and expands their use in future agreements. Finally, constructive interaction among all stakeholders to properly execute MEAs expedites the pursuit of their common interest, which is patients' improved and accelerated access to drugs.