1. Background
The genus Artemisia, with more than 500 species, is the largest member of the Compositae (Asteraceae) family, distributed in different areas of Asia, Europe, and North America (1). The inhibitory effects of essential oils, crude extracts, and different fractions of Artemisia against the growth of cancer cell lines have been reported (2). The studies have demonstrated that secondary metabolites, mainly flavonoids, terpenoids, and coumarins, are responsible for the cytotoxic effects in the genus Artemisia (3-6). Essential oil of A. persica and A. turcomanica inhibited the MCF-7 cell line time and dose-dependently (7). Essential oil of A. annua showed cytotoxic activity on SMMC-7721 cells via inducing apoptosis (8). Essential oil of A. lavandulaefolia exhibited antiproliferative activity against HeLa cells by activating caspase 3 (9). Essential oil of A. vulgaris induced apoptosis in HL-60 leukemic cells and showed anticancer activity with cytochrome C releasing and activating caspases-3, 8, and 9 (10). Dichloromethane extract of A. biennis showed the most effective cytotoxic activity on K562 and HL-60 cancer cell lines by apoptosis induction (11). Similar results have been obtained for A. ciniformis, A. turanica, and A. armeniaca on human cancer cell lines (12-14). Select fractions obtained from A. ciniformis and A. biennis were assessed for their cytotoxic activity on B16/F10, PC3, and MCF-7 cells and revealed promising results as potential sources of cytotoxic phytochemicals (15). A study on extracts of three samples of A. khorassanica for their cytotoxic activity against AGS, HELA, HT-29, and MCF-7 exhibited that dichloromethane extract was most effective on cancer cell lines. Furthermore, MCF-7 was the most sensitive among other cell lines (16).
Artemisia haussknechtii Boiss., with Persian names Dermaneh Zagrosi and Dermaneh Sakhreie, is one of the 34 species of Artemisia that grows in the west of Iran (17). A study on its crude extracts and essential oils has demonstrated in vitro antifungal (18), antibacterial, antioxidant (19), and insecticidal activities (20). Also, camphor and 1,8-cineole have been reported as significant constituents in the volatile oil. Other components included cis-davanone, 4-terpineol, linalool, beta-fenchyl alcohol, and borneol (21, 22).
2. Objectives
Due to the importance of the Artemisia genus for inhibiting the growth of different cancer cell lines, this study investigated the cytotoxicity effect of their crude extract and fractions. In addition, the purification and structure elucidation of compounds responsible for cytotoxic effects of the most effective fraction were done.
3. Methods
3.1. Plant Material
The aerial parts of A. haussknechtii Boiss. were collected from Kurdistan province (Salavat Abad mountain pass), Iran, in November 2018. The sample was identified by Alireza Dolatyari. The voucher specimen (No. 320) was deposited in the Herbarium of the Iranian Biological Research Center, Tehran, Iran.
3.2. Extraction and Fractionation
Shade-dried and grained aerial parts of A. haussknechtii (800 g) were macerated by 70% MeOH/H2O (4 × 8 L × 3 days). The crude extract (100 g) was fractionated via liquid-liquid extraction. The crude extract was dispersed in H2O, and three solvents (petroleum ether (40:60), dichloromethane, and n-butanol) were used to yield 3, 11, and 65 g of each fraction, respectively. All samples were concentrated using a rotary evaporator under reduced pressure and temperature below 45°C.
3.3. General Experimental Procedures
The chromatographic process was performed using an analytical HPLC system (Shimadzu Lc 10A, Tokyo, Japan) equipped with a photodiode array detector (PDA) and an Ascentis® column (250 × 10 mm i. d., 5 μm). Pre-coated TLC aluminum sheets (silica gel 60 F254, Merck, Germany) were applied for monitoring all subfractions under a UV cabinet (254 and 365 nm) using an anisaldehyde sulfuric acid reagent to combine similar subfractions. The NMR spectra were recorded on a Varian INOVA (500 MHz) spectrometer using CDCl3 and DMSO-d6 as the solvent. The ESIMS data were obtained from Triple Quad LC/Mass (Agilent technologies 6410, USA). All solvents used in the extraction and purification process were purchased from Merck (German) and Chem lab (Belgium) with laboratory and HPLC grades.
3.4. Cell Culture Conditions
Human breast adenocarcinoma MCF-7 cell line (ATCC No. HTB-22) was purchased from the Pasteur Institute (Tehran, Iran) and maintained at 37ºC in a humidified atmosphere (90%) containing 5% CO2. The cells were seeded in the RPMI-1640 medium with 10% (v/v) heat-inactivated fetal bovine serum, 100 U/mL penicillin, and 100 mg/mL streptomycin (Gibco, USA).
3.5. Cytotoxicity Assay
The cytotoxic effects of crude extract and fractions on the MCF-7 cell line were evaluated by MTT assay. First, 180 µL of medium containing MCF-7 cells was seeded on 96-well plates (approximately 5 × 103 cells/well). Each well was treated with a specific concentration of samples (20 µL) after 24 h incubation (37°C and 5% CO2). All samples and media were removed 48 h later. Then, 20 µL MTT (5 mg/mL) was added to each well, and the plates were further incubated for 3 h at 37°C. Finally, 100 µL of dimethyl sulfoxide (DMSO) was added to each well to dissolve formazan crystals. All experiments were repeated three times for each concentration. The optical density (OD) was measured on a microplate reader (BioTek Instruments, USA) at 570 nm. The inhibitory rate of cell proliferation was calculated according to the following formula: Growth inhibition (%) = (Acontrol – Atreated) / Acontrol × 100
Where Acontrol is the absorbance of the control group (containing no samples) and Atreated indicates absorbance of the sample at 570 nm. The results were reported for each sample as IC50 (µg/mL).
3.6. Purification and Structure Elucidation
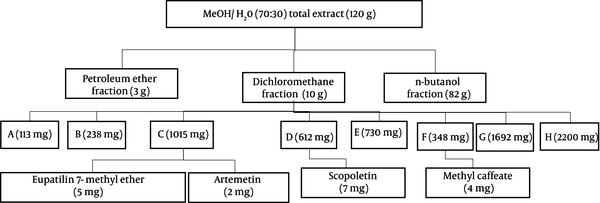
The chromatographic process of the most effective fraction based on cytotoxic results was performed with column chromatography (CC) (L = 100 cm and D = 5.5 cm). A portion of dichloromethane fraction (10 g) moved on silica gel normal phase (230 - 400 mesh ASTM, Merck, Germany) column and eluted with the increasing amount of ethyl acetate in chloroform (1:9 - 10:0) to get eight subfractions (A-H) (Figure 1).
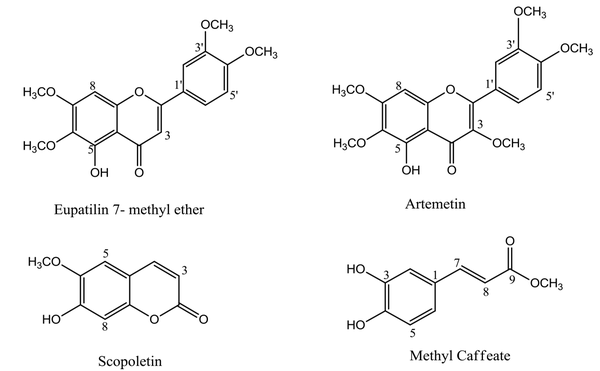
Further purification of C was performed on TLC sheets and mobile phase chloroform: ethyl acetate (9:1) to get compounds 1 and 2 (Rf = 0.65, 5 mg, and Rf = 0.72, 2 mg, respectively). Purification of D by analytical HPLC (mobile phase: 0 - 40, MeOH from 20% to 80% in H2O; 40 - 45 min, 80% MeOH in H2O; 45 - 46 min, 80% MeOH in H2O to 100% MeOH; 46 - 50 min, MeOH 100%; 50 - 52 min, MeOH 100% back to 20% MeOH in H2O) yielded compound 3 (7 mg, Rt = 13.2 min). Also, purification of F performed by the same gradient solvent system yielded compound 4 (4 mg, Rt = 25 min) (Figure 1). Structure elucidation of the isolated compounds was carried out by spectrum data obtained from 1D-NMR (13C and 1H-NMR) and mass (ESIMS). All data were in good agreement with those reported in the literature. The chemical structures of isolated compounds are shown in Figure 2.
4. Results and Discussion
This study evaluated the cytotoxic effects of crude extract and three fractions on MCF-7 cells by MTT assay. The results showed that the dichloromethane fraction had the most effective cytotoxic effect (Table 1).
| Total Extract | Petroleum Ether | Dichloromethane | n-Butanol |
|---|---|---|---|
| 987.98 ± 4.23 | 377.18 ± 3.36 | 297.17 ± 7.99 | 1094.85 ± 9.24 |
IC50 Values (µg/mL) for Cytotoxic Effects of Crude Extract and Fractions on MCF-7 Cell Line (Mean ± SD, n = 3)
Nonpolar and semipolar extracts and fractions can be good candidates for cytotoxicity studies of the Artemisia genus. The polarity of the solvent plays a vital role in the extraction process. Dichloromethane is an organic solvent with a low polarity that can extract secondary metabolites with similar polarities, like terpenoids, coumarins, alkaloids, and polymethoxylated flavonoids. These types of phytochemical compounds can be responsible for cytotoxic effects on cancer cell lines (11, 12, 23, 24). Also, some mechanisms have been proposed for exerting cytotoxic effects of dichloromethane fractions and their isolated compounds, such as increasing the level of Bax protein, cleavage of PARP protein, and formation of DNA fragments (11, 14). Phytochemical analysis of the fraction with the most cytotoxic effects led to the isolation of eupatilin 7-methyl ether (1), artemetin (2), scopoletin (3), and methyl caffeate (4).
4.1. Spectroscopic Data of Isolated Compounds
Compound (1) 5-Hydroxy-3',4',6,7-tetramethoxyflavone (eupatilin 7-methyl ether): ESI-MS (m/z): 359.3 [M+H]+, yellow needle crystals, molecular formula C19H18O7; 1H NMR (500 MHz, CDCl3) δ 12.75 (1H, s, 5-OH), 7.52 (1H, dd, J = 8.5, 2.2 Hz, H-6'), 7.34 (1H, d, J = 2.1 Hz, H-2'), 6.98 (1H, d, J = 8.6 Hz, H-5'), 6.59 (1H, brs, H-8), 6.55 (1H, brs, H-3), 3.99 (3H, s, 7-OMe), 3.98 (3H, s, 3'-OMe), 3.96 (3H, s, 4'-OMe), 3.93 (3H, s, 6-OMe). 13C NMR (75 MHz, CDCl3) δ 182.78 (C-4), 164.14 (C-2), 158.91 (C-7), 153.40 (C-9), 153.24 (C-5), 152.47 (C-4'), 149.52 (C-3'), 132.84 (C-6), 123.97 (C-1'), 120.26 (C-6'), 111.32 (C-5'), 108.97 (C-2'), 106.33 (C-10), 104.78 (C-3), 90.77 (C-8), 61.05 (6-OMe), 56.50 (3'-OMe), 56.28 (4'-OMe), 56.04 (7-OMe), (25-29).
Compound (2) 5-hydroxy 3,3',4',6,7- pentamethoxy-flavone (artemetin): ESI-MS (m/z): 389.3 [M+H]+, yellow crystals, molecular formula C20H20O8; 1H NMR (500 MHz, CDCl3) δ 12.61 (1H, brs, 5-OH), 7.73 (1H, dd, J = 8.5, 2.1, H-6'), 7.69 (1H, d, J = 2.1, H-2'), 6.99 (1H, d, J = 8.6, H-5'), 6.50 (1H, s, H-8), 3.97 (3H, s, 4'-OMe), 3.97 (3H, s, 3'-OMe), 3.96 (3H, s, 7-OMe), 3.92 (3H, s, 6-OMe), 3.87 (3H, s, 3-OMe). 13C NMR (75 MHz, CDCl3) δ 178.89 (C-4), 158.78 (C-7), 155.94 (C-2), 152.8 (C-5), 152.33 (C-9), 151.46 (C-4'), 148.85 (C-3'), 138.81 (C-3), 132.35 (C-6), 123.09 (C-1'), 122.32 (C-6'), 111.36 (C-2'), 110.85 (C-5'), 106.63 (C-10), 90.32 (C-8), 60.9 (6-OMe), 60.22 (3-OMe), 56.33 (7-OMe), 56.30 (3'-OMe),56.01 (4'-OMe), (30-32).
Compound (3) 6-methoxy-7-hydroxycoumarin (scopoletin): ESI-MS (m/z): 193.1 [M+H]+, beige needle crystal, molecular formula C10H8O4; 1H NMR (500 MHz, CDCL3) δ 7.59 (1H, d, J = 9.5 Hz, H-4), 6.92 (1H, s, H-5), 6.85 (1H, s, H-8), 6.27 (1H, d, J = 9.5 Hz, H-3), 6.11 (1H, brs, 7-OH), 3.96 (3H, s, 6-OMe). 13C NMR (75 MHz, CDCl3) δ 161.55 (C-2), 150.44 (C-7), 149.82 (C-9), 144.14 (C-6), 143.38 (C-4), 113.63 (C-5), 111.64 (C-10), 107.62 (C-3), 103.18 (C-8), 56.58 (6-OCH3), (33, 34).
Compound (4) methyl caffeate: ESI-MS (m/z): 193.1 [M-H]-, yellow needle crystals, molecular formula C10H10O4; 1H NMR (500 MHz, DMSO-d6) δ 7.48 (1H, d, J = 16 Hz, H-7), 7.05 (1H, J = 2.2 Hz, H-2), 7.00 (1H, dd, J = 8.1, 2.0 Hz, H-6), 6.76 (1H, d, J = 8.1 Hz, H-5), 6.27 (1H, d, J = 15.9 Hz, H-8), 3.68 (3H, s, OCH3). 13C NMR (75 MHz, DMSO-d6) δ 167.08 (C-9), 148.48 (C-4), 145.62 (C-3), 145.19 (C-7), 125.50 (C-1), 121.4 (C-6), 115.73 (C-5), 114.84 (C-2), 113.71(C-8), 51.24 (OCH3) (35, 36).
Polymethoxyflavones (PMFs) belong to flavonoid compounds with two or more methoxy groups in the structure, imposing anti-inflammatory and anticancer pharmacological effects. Acetylation and hydroxylation of PMFs have been shown to reduce lipophilicity and improve their pharmacological effects. The in vitro and in vivo studies have revealed potent cytotoxic activity of these compounds in all three stages of cancer cells formation (initiation, promotion, and progression) (37). Eupatilin 7-methyl ether and artemetin are polymethoxyflavones that have been found in various Artemisia species (31, 38-43). Eupatilin 7-methyl ether has shown moderate cytotoxic effects against Hep-G2, MCF-7, and HeLa cell lines with IC50 values of 28.3, 9.5, and 10.1 µg/mL, respectively (29). While up to 100 µM of this compound had no cytotoxic effect, 200 and 300 µM have exhibited significant cytotoxic effects against human normal fibroblast cells (BJ-1) (44). Eupatilin 7-methyl ether isolated from A. argyi has shown a significant protective effect on contrast-induced nephrotoxicity by iodixanol in LLC-PK1 cells (38). Scopoletin belongs to coumarin compounds and has been found in A. annua and A. incisa (45, 46). Many pharmacological effects have been reported for scopoletin, such as antibacterial, antifungal, antioxidant, and antiproliferative (47). Scopoletin, isolated from the ethyl acetate extract of A. argyi, showed outstanding antiproliferative activity against CCRF-CEM leukemia cells with an IC50 of 2.6 μM (48). Methyl caffeate has been reported from A. integrifolia, A. argyi, and A. annua (49-51). In vitro studies have shown significant cytotoxic activity of cinnamic acid derivatives, like methyl caffeate, on different cancer cell lines (IC50 ≤ 5 µg/mL) (52).
4.2. Conclusions
To the best of our knowledge, this is the first report on cytotoxic activity and phytochemical analysis of total extract and fractions of A. haussknechtii, which resulted in the isolation of compounds with cytotoxic potential on cancer cell lines. Various compounds isolated from this plant, especially polymethoxyflavones, can be considered for further studies.