1. Background
Euphorbiaceae is one of the most prominent flowering plants in the tropics consisting of more than 300 genera and 8,000 species (1). The genus Euphorbia includes more than 2,000 species in Asia and other parts of the world. About 70 species are found in Iran, including 17 native species (2). In Iran, Euphorbia aleppica is found in both West and East Azerbaijan provinces around Lake Urmia, Kermanshah, Qasr-e-Shirin, Biston, Ilam, and Arak (3). Razes in Al-Hawi has introduced Euphorbia to treat sciatica, insect bites, headaches, paralysis, and numbness and eliminate warts, and as a poultice to relieve nerve pain and rheumatism (4). Euphorbia species are cytotoxic by inhibiting cell proliferation, apoptosis induction, and anti-angiogenesis (1, 5). Cytotoxic effects of E. kopetdaghi against human ovarian carcinoma (OVCAR-3), and human bladder carcinoma (EJ138) (6), E. denticulata against human prostate cancer (DU-145) (7), and E. turcomanica against cervical cancer cell line (HeLa) were reported (8).
Euphorbia aleppica grows in the northern hemisphere, including Iran. In 1995, premyrsinane and myrsinol type diterpenoids were identified from this plant collected from Irbid in Jordan and Istanbul in Turkey (8-10).
2. Objectives
Due to the rare reports on the structures of isolated compounds, this study aimed to isolate and identify other bioactive diterpenoid chemical structures so that they can be further studied in the future through secondary bioassays and possibly semisynthetic approaches (Figure 1).
3. Methods
3.1. Materials
Optical rotations were obtained by an ATAGO automatic compact polarimeter POL 1/2 (Atago Corp., Tokyo, Japan) equipped with a sodium lamp (589 nm). The NMR spectra were taken on Bruker 400 spectrometer using deuterated CHCl3 (δH 7.24/ δC 77.23) signals as reference. The HR-ESI-MS was acquired on a Micromass Q-TOF2 (an Applied Biosystem API 2000 triple-quadrupole) mass spectrometer. Silica gel (70 - 230 and 230 - 400 mesh, Merck™) and Sephadex LH-20 (Pharmacia Biotech AB, Uppsala, Sweden) were used for column chromatography.
3.2. Plant Material
Euphorbia aleppica (L.) Fourr., synonyms: E. condensate Fisch. ex M. Bieb., E. juncoides (Haw.) Sweet, E. juncea Aiton, and E. pinifolia Willd., from the Euphorbiaceae family were collected in Jun 2019 from Kuhdasht, Lorestan, Iran. Mahboobeh Khatamsaz determined it, and the voucher specimen (SAM-4004) was stored in the Samsam-Shariat Herbarium, Isfahan University of Medical Sciences.
3.2.1. Extraction and Isolation
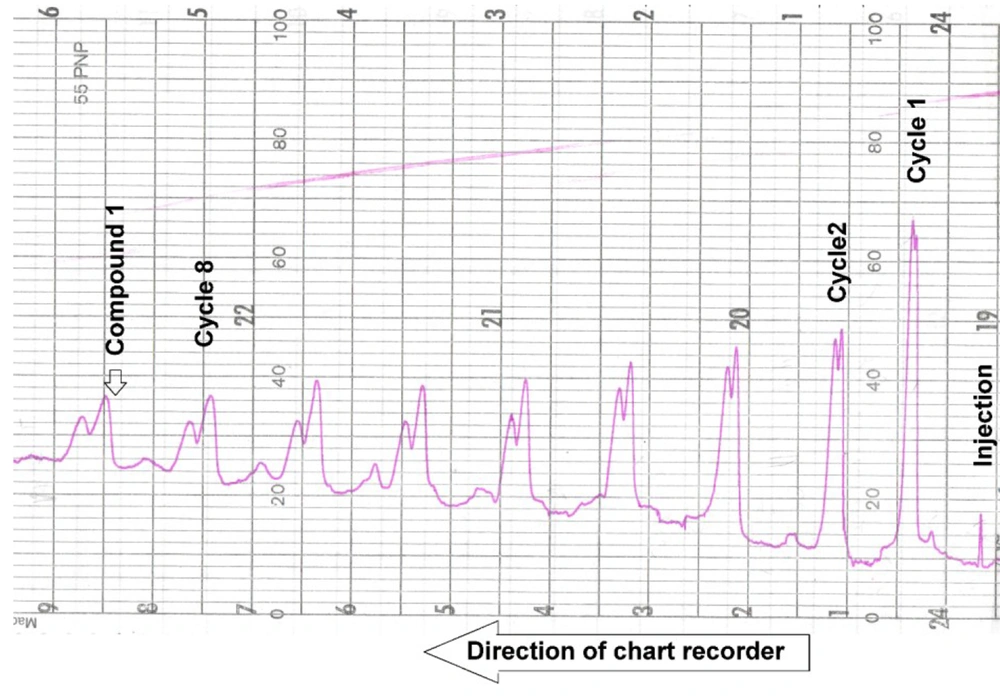
Plant material (2.2 kg) was extracted in CH2Cl2: acetone (2: 1; 10 L × 3) at room temperature for three successive weeks. A rota-evaporator concentrated the extract at 40°C to 250 g (yield 11.3%). It was filtered through a cartridge of reverse silica gel by MeOH: H2O (70: 30) to remove fatty contents. The resulting extract was concentrated (123 g) and applied to an open silica gel column (63 - 200 µm; 70 × 400 mm i.d) using a gradient system of hexane: Ethyl acetate by increasing polarity (11). Based on the TLC profile ( hexane: Ethyl acetate 70: 30) visualized with cerium sulfate reagent (1%) in 5% H2SO4 and initial 1H-NMR spectra, we selected fractions Af-5-3 (1.36 g) and Af-5-4 (1.66 g) showing dark-brown spots on TLC with retention factor of 0.2 - 0.6 and NMR resonances δH of 0.5 - 1.6 related to terpenoids, δH 1.8 - 2.2 related to singlet methyls of acetyl esters, and δH 3 - 6 ppm pertaining to a couple of oxymethins indicating a polyol core characteristic in diterpene polyesters (11, 12). Selected fractions were injected into a Sephadex LH-20 column (30 × 300 mm i.d) using hexane: acetone: methanol (30: 10: 60) as the mobile phase. Based on the TLC profile, diterpene rich subfractions AF-1-9-3 (0.63 g) and Af-10.3 (0.34 g) were applied to an open glass silica gel column (40 - 63 µm; 20 × 900 mm i.d) using hexane: Ethyl acetate (85: 15, 300 cc; 80: 20, 600 cc) to yield Af-31-1 and Af-42-2 as semi-pure compounds. However, Af-31-1 was a mixture of two close compounds and difficult to separate. It was injected into a closed-loop recycling HPLC system attached to a YMC-Pack Sil column (20 × 250 mm i.d) equipped with refractive index and UV detector at 250 nm using hexane: Ethyl acetate (70: 30) at a flow rate of 3 mL/min. After passing through the HPLC column and detectors, selected peaks would be directed by a three-way valve to the suction part of the HPLC pump and eluted again in the column in a closed loop continuously until the appropriate separation was achieved. As shown in Figure 2, two peaks overlapped in the first cycle, but they were gradually resolved and separated from each other in the following recycling stages. Finally, after nine cycles from the injection, we collected Af-50-1 (6 mg) as compound 1, while Af-50-2 (2.4 mg) was put aside due to its low quantity (12). However, Af-42-2 was submitted to the same HPLC system and purified after three cycles to yield compound 2 (4.8 mg) in a pure state.
HPLC chromatogram of compound 1 in a closed-loop recycling HPLC system on semi-preparative YMC-Pack Sil (20 × 250 mm i.d), using hexane: Ethyl acetate (70: 30) coupled with RI detector on a chart recorder. From right to left, two peaks overlapped in cycle 1, but they were gradually resolved and separated after each cycle. Finally, after nine cycles from the injection, we collected the peaks.
3.3. Cell Culture
We prepared MCF-7 and MDA-MB 231 cancer cells from the Pasteur Institute of Iran. They were cultured in a DMEM medium with 10% FBS, penicillin, and streptomycin (100 U/mL + 100 μg/mL) and maintained in an incubator at 37°C with 5% CO2 and 95% humidity.
3.4. Cell Viability Assay
The MCF-7 and MDA-MB 231 breast cancer cells (5,000 cells in each well) were seeded in a 96-well plate and incubated at 37°C. We treated the incubated cells with compounds 1 and 2 (0.1, 1, 10, 100, and 200 µM) for two days, while the vehicle for each concentration was the negative control. Taxol (0.1, 1, 10, and 100 µM) was used as the positive control. After 48 h, an MTT solution of 0.5 mg/mL (20 µL) was added to the wells, then incubated at 37°C for four hours. Then, we discarded the supernatants, added 100 µL DMSO to each well, and read the optical density at 570 nm by a BioTek microplate reader (Winooski, VT, USA) (13).
3.5. Statistical Analyses
Data were expressed as mean ± SD. Statistical analysis was done by the one-way ANOVA, followed by Dunnett’s post hoc test. The p values less than 0.05 compared to the control showed statistically significant differences.
4. Results
Euphorbia aleppica was phytochemically analyzed using a repeated chromatographic method, which detected two diterpene compounds as follows (Figure 1):
Compound 1: Colorless oil; [α]D25 + 8.83 (c 0.6 CHCl3); for 1H and 13C NMR data, see Table 1; UV (CHCl3) λmax (log ε) 251.2 (2.932); HR-ESI-MS m/z 597.2692 [M + Na]+, calc. for C31H42O10Na, 597.2670.
Compound 2: Colorless oil; for 1H and 13C NMR data, see Table 1; HR-ESI-MS m/z 699.2998 [M + Na]+ calc. for C36H50O12Na+, 699.2987.
| Position | Compound 1 | Compound 2 | ||
|---|---|---|---|---|
| δH (mult., J in Hz) | δC | δH (mult., J in Hz) | δC | |
| 1 a | 3.36 (dd, J = 15.0, 9.8) | 42.2 | 1.80 (dd, J = 15.2,10.0) | 44.83 |
| 1 b | 1.42 - 1.56 (dd, J = 15.0, 5.3) | 2.40 (dd, J = 15.3, 8.5) | ||
| 2 | 2.11 - 2.19 (m) | 35.7 | 2.0 - 2.28 (m) | 36.54 |
| 3 | 5.18 (dd, J = 4.2, 3.9) | 77.5 | 5.11 (t, J = 3.8) | 77.23 |
| 4 | 2.45 (dd, J = 10.7, 3.9) | 53.7 | 3.14 (dd, J = 10.7, 3.6) | 51.5 |
| 5 | 5.89 (d, J = 10.7) | 67.9 | 5.86 (d, J = 10.7) | 66.92 |
| 6 | --- | 54.1 | --- | 56.3 |
| 7 | 4.72 (dd, J = 8.8, 3.6) | 75.3 | 5.06 (dd, J = 10.9,3.2) | 71.75 |
| 8 a | 1.95 (dd, J = 7.8, 3.6) | 24 | 1.23 - 1.27 (m) | 25.11 |
| 8 b | 1.56 - 1.46 (m) | 62.96 | 1.86 - 1.94 (m) | 63.00 |
| 9 | 1.02 - 0.93 (m) | 23.3 | 0.94 - 1.04 (m) | 23.58 |
| 10 | --- | 18.7 | --- | 19.22 |
| 11 | 0.75 (dd, J = 9.7, 5.9) | 17.7 | 0.84-0.93 (m) | 18.31 |
| 12 | 2.74 (d, J = 5.9) | 40.5 | 2.56 (d, J = 7.4) | 39.97 |
| 13 | --- | 90.2 | --- | 89.39 |
| 14 | --- | 202.7 | 5.658 (s) | 73.08 |
| 15 | --- | 88.8 | --- | 88.17 |
| 16 | 0.88 (d, J = 6.9) | 15 | 0.83 (d, J = 6.7) | 14.45 |
| 17 | 4.14 (d, J = 9.6) | 72.7 | 6.59 (s) | 97.91 |
| 4-OH | 3.7 (d, J = 9.6) | - | ||
| 18 | 1.06 (s) | 28.7 | 1.32 (s) | 25.21 |
| 19 | 1.08 (s) | 16 | 1.03 (s) | 15.87 |
| 20 | 1.52 (s) | 20.5 | 1.08 (s) | 27.83 |
| 3-OAc | --- | 170.5 | --- | 170.18 |
| 2.10 (s) | 21.1 | 1.8 | 21.12 | |
| 5-O-tig | --- | 165.8 | --- | 165.73 |
| 2' | --- | 128.8 | --- | 128.83 |
| 3' | 6.75 (q, J = 6.7) | 138.8 | 6.89 (q , J = 6.7) | 138.41 |
| 4' | 1.75 (s) | 14.3 | 1.804 (d , J = 6.7) | 14.63 |
| 5' | 1.76 (s) | 12.2 | 1.79 (s) | 12.01 |
| 7-OAc | --- | 170.2 | --- | 169.69 |
| 2.13 (s) | 21.4 | 2.08 | 21.41 | |
| 14-OAc | --- | --- | 169.69 | |
| --- | 2.2 | 20.94 | ||
| 15-OAc | --- | 170.8 | --- | 170.74 |
| 1.84 (s) | 21.4 | 2.17 | 22.7 | |
| 17-OAc | --- | --- | 169.13 | |
| --- | 2.08 | 21.07 | ||
a δ values were established from HMBC, COSY, and HSQC.
b Overlapping with other signals
4.1. Cell Proliferation Assay
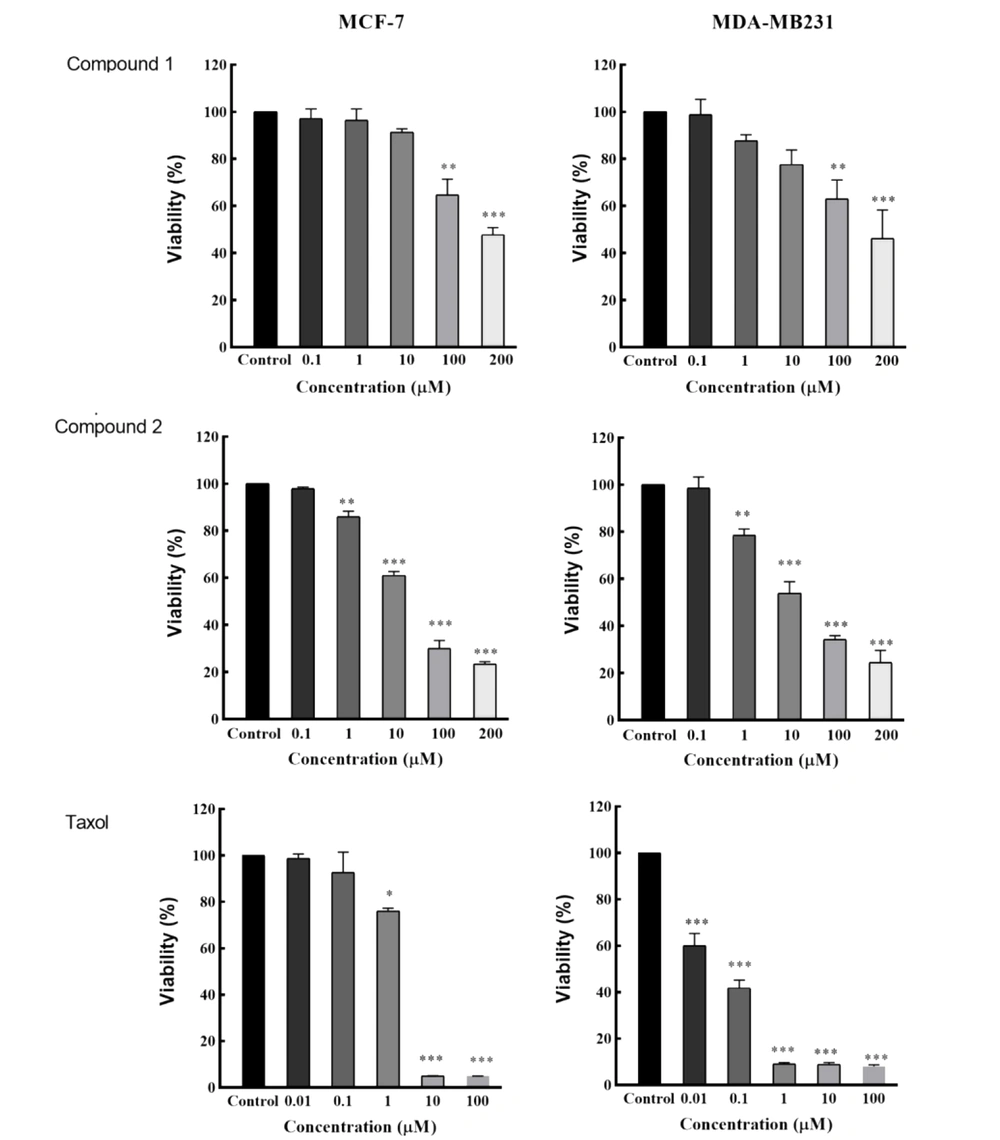
The isolated compounds were evaluated against breast cancer cells to check cytotoxicity (Figure 2). Compound 1 showed moderate cytotoxicity with IC50 values greater than 100 μM, while compound 2 showed potent cytotoxicity with IC50 values of 17.6 ± 1.2 and 16.7 ± 1.5 µM against MCF-7 and MDA-MB 231, respectively. As a standard drug, Taxol showed cytotoxicity with IC50 values of 4.36 ± 0.12 and 0.05 ± 0.01 µM against MCF-7 and MDA-MB 231, respectively (Figure 3).
The effect of compounds 1 and 2 on cell proliferation inhibition in breast cancer cell lines. Cells were treated with different concentrations of compounds 1 and 2 for 48 h, and the MTT assay assessed proliferation. Vehicles and Taxol were used as negative and positive controls, respectively. Data (mean ± SD) were calculated as the percent of corresponding control values. *P < 0.05, **P < 0.01, and ***P < 0.001 are significantly different from control cells. Statistical analysis was performed using ANOVA. All measurements were done in triplicate.
5. Discussion
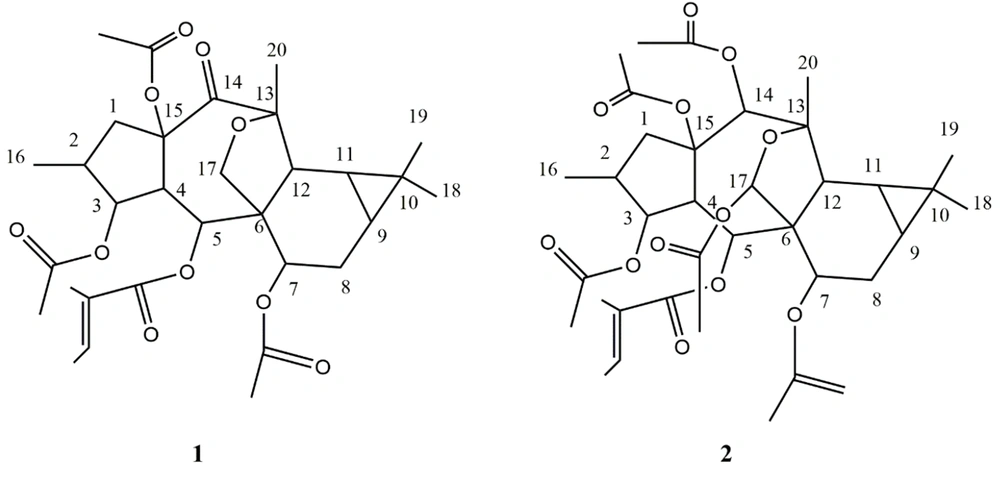
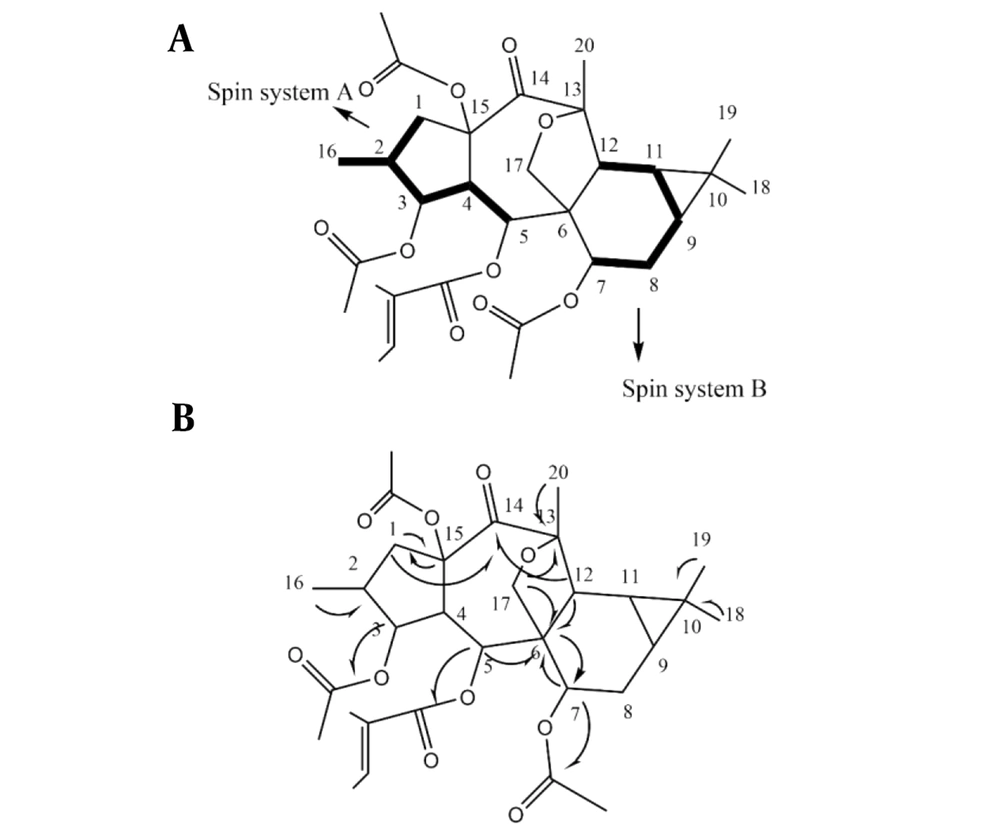
A closed-loop recycling HPLC system separated compounds 1 and 2 on a normal phase column. The NMR spectra of the isolated compounds resembled diterpene polyesters found in Euphorbia species. The 1H- and 13C-NMR spectra of compound 1 showed residuals of four ester groups. Also, δC 165.8, 128.8, 138.1 (δH: 6.75, q, J = 6.7 Hz), 14.3 (δH: 1.75, s), and 12.2 (δH: 1.76, d, J = 6.7 Hz) belonged to the tigliate group (10). Besides, δC 170.5, 21.0 (δH: 2.10, s), 170.2, 21.4 (δH: 2.13, s), 170.8, and 21.4 (δH: 1.84, s) belonged to three acetate groups (12). Without these four ester groups attached to the main structure, based on hydrogen and carbon information in the 1H-, 13C-NMR, and DEPT spectra, the polyole core consisted of 20 carbons, including four methyl groups, three methylene groups (one attached to oxygen), eight methine groups (three attached to oxygen), and five unprotonated carbons (one ketone and two quaternary oxycarbons), indicating a macrocyclic diterpene. The 1H-NMR spectrum showed the resonances of one doublet methyl group (δH: 0.88, d, J = 6.9, Me-16), three singlet methyl groups (δH: 1.06, s, Me-18 / 1.08, s, Me-19 / 1.52, s, Me-20), two simple methylenes (δH: 3.36, dd, J = 15.0,9.8 Hz / 1.42 - 1.50, dd, J = 15.0, 5.3 Hz related to H-1a, b, and δH: 1.95, dd, J = 7.8,3.6 / 1.46-1.56, m, related to H-8a, b), one oxygenated methylene (δH: 4.14, d, J = 9.6 Hz / 3.79, d, J = 9.6 Hz related to H-17a, b), five simple methines including H-2 (δH: 2.11 - 2.13, m), H-4 (δH: 2.45, dd, J = 10.7, 3.9 Hz), H-12 (δH: 2.74, d, J = 5.9 Hz), and two upfield methines (δH: 0.75, dd, J = 9.7, 5.9 Hz / 0.93 - 1.02, m) related to H-9 and H-11 on the triangular ring, as well as three oxymethins: H-3 (δH: 5.18, dd, J = 4.2, 3.9 Hz), H-5 (δH: 5.89, d, J = 10.7 Hz), and H-7 (δH: 4.72, dd, J = 8.8, 3.6 Hz). The 1H-1H COSY couplings determined two spin systems, including A (H1 - H5) as CH2 – CH(CH3) - CHO - CH - CHO and B (H7 - H12) as CH - CH2 - CH - CH - CH. A long-range correlative HMBC spectrum was used to determine the connection of spin components with quaternary carbons, end terminal segments, and locate ester groups. Spin systems A and B were connected through quaternary carbons C-6, C-15, C-10, C-13, and C-14 by HMBC correlations of C-6 / H-5, H-7, H-12; C-15 / H-1a, b, C- 10 / H-11; C-13 / H-12; C-14 / H-1b, H-12. HMBC of C-10/Me-19, Me-18; C-13/Me-20, H-17; C-6/H-17 determined the position of singlet methyls 18, 19, 20 and oxymethylene C-17. HMBC of δC 165.8/δH 5.89 (H-5); δC 170.5/δH 5.89 (H-3); δC 170.2/δH 4.72 (H-7) located the tigliate group on C-5 and two acetate esters on C-3 and C-7. One of the acetate groups showed no HMBC with oxymethines; therefore, it was placed on the remaining oxycarbon C-15 resonated at δC 90.2 ppm (Figure 4).
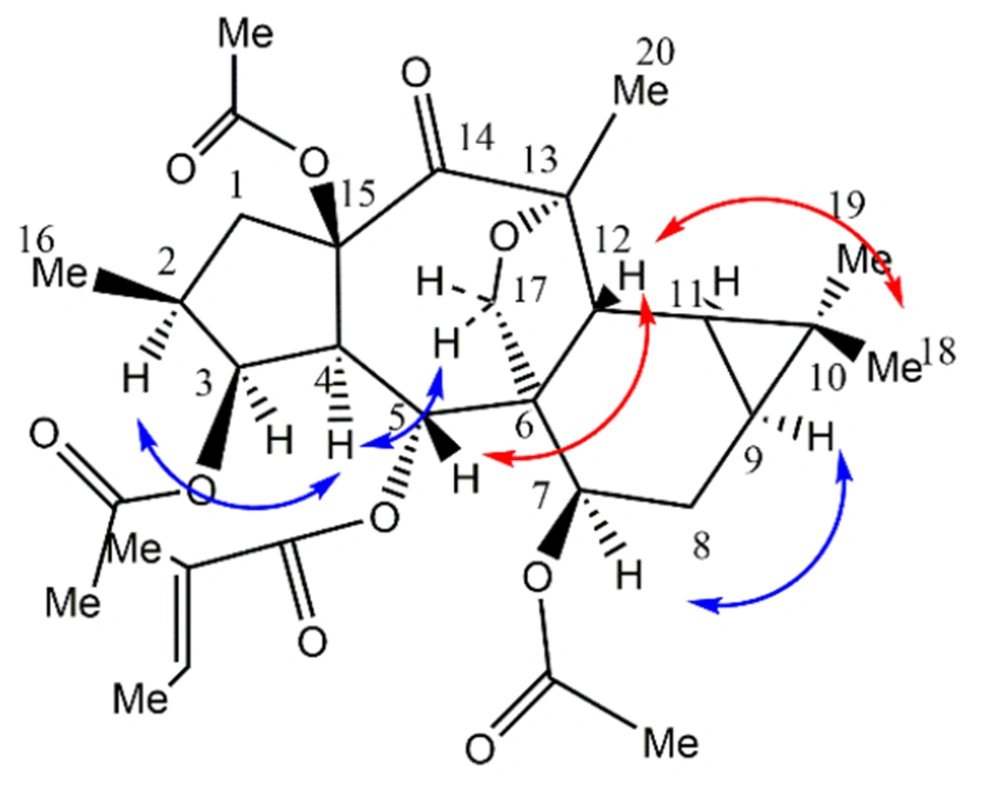
The stereochemistry was done through the NOESY spectrum and analyzing the coupling constants. In the NOESY experiment, taking H-4 in alpha and 15-OR in beta orientation, typical in premyrsinane Euphorbia diterpenes, NOEs of H-4/H-2, H-17a as well as the small coupling constant of J3,4 = 3.9 Hz (determinative of cis-relationship of H-4/H-3) determined alpha orientation of H-2, H-3, and five-membered 13(17)-epoxy ring. Relative large coupling constant of J4,5 = 10.7 Hz determined H-4 and H-5 trans conformation; thus, the beta orientation of H-5 and alpha orientation of 5-O-tigliate. NOEs of H-5/ H-12; H-12/Me-18 determined the beta orientation of H-12 and Me-18, thus the triangular ring’s beta orientation. NOEs of H-9/H-7 determined H-7, H-9, and H-11 alpha orientation. Me-20 showed NOE correlation with H-12 and H-11, indicating its equatorial direction. Finally, according to the information obtained from NMR data, as well as the exact mass [M + Na]+ of 597.2692 calculated for C32H46O10 + Na+ (calcd. 597.2670), the structure of compound 1 was suggested as 3,7,15β- triacetyl-5α-tigliate-13(17)-α-epoxy-14-oxopremyrsinane as a newly described compound (Figure 5).
In compound 2, the NMR spectra determined six ester groups. Resonances of δC 165.73, 128.83, 138.41 (δH: 6.89, q, J = 6.7 Hz), 14.63 (δH: 1.8, s), and 12.01 (δH: 1.79 s) belonged to the tigliate group (10). δC 170.74, 22.7 (δH: 2.17, s), δC 170.18, 21.12 (δH: 1.8, s), δC 169.69, 21.07 (δH: 2.20, s), δC 169.69, 21.41 (δH: 2.08, s), and δC 169.13, 21.13 (δH: 2.08, s) belonged to five acetate groups. Without esters attached to the main skeleton, based on the 13C-NMR and DEPT, the core consisted of 20 carbons, including four methyl carbons, two methylene carbons, 10 methine carbons (four oxymethines and one hemiacetal), and four quaternary carbons, including two oxycarbons. The 1H NMR spectrum showed resonances of one dublet methyl group (δH: 0.83, d, J = 6.7 Hz, Me-16), three singlet methyl groups (δH: 1.32, s, Me-18 / 1.03, s, Me -19 / 1.08, s, Me-20), two methylene groups [δH: 1.80 (dd, J = 15.2, 10.0 Hz)/2.40 (dd, J = 15.3, 8.5 Hz) related to H-1a, b and δH: 1.23 - 1.27 (m)/1.86 - 1.94 (m) related to H-8a, b], five simple methine groups including three simple methines: H-2 (δH: 2-2.28, m), H-12 (δH: 2.56, d, J = 7.4 Hz), H-4 (δH: 3.14, dd, J = 10.7, 3.6 Hz), and two in the upfield area resonated at δH: 0.94 - 1.04 (m) and 0.84 - 0.93 (m) related to H-9 and H-11 on a triangular ring, four oxymethine groups at δH: 3.14 (dd, J = 10.7, 3.6 Hz) related to H-3, at δH: 5.86 (d, J = 10.7 Hz) related to H-5, at δH: 5.06 (dd, J = 10.9,3.2 Hz) related H-7, at δH: 5.658 (s) related to H-14 and one hemiacetal group at δH: 6.59 (s) related to H-17. Finally, according to the information obtained from NMR, as well as the exact mass [M + Na]+ of 699.2998, the structure of compound 2 was determined as 3, 7, 14, 15, 17 - pentaacetyl-5-tigliate-13 (17)-hemiacetal premyrsinane as a pre-described compound (9). It was similar to compound 1 differed in lack of the ketone group at C-14 and two other acetate esters at C-14 and C-17.
In the MTT assay, compound 2 exhibited a potent cytotoxic effect in a dose-dependent manner comparable with compound 1 with moderate cytotoxicity against MDA-MB231 and MCF-7 cancer cells. It is probably because of the 14-O-acetyl group instead of the ketone group at C-14 and the presence of the 3(17)-acetylated hemiacetal five-membered ring instead of 13(17)- epoxy ring in compound 1.
These results agree with the literature on the cytotoxicity activity of premyrsinanes. In a study conducted by Xiao et al., two simple 3, 5, 15-trihydroxy-14-oxopremyrsinane structures with three ester functions were semi-synthesized by the bioinspired Fe(acac)3-catalyzed method from Euphorbia factor L3 with lathyrane structure. Premyrsinanes showed potent cytotoxic activity against the 4T1 breast cancer cell line with IC50 values of 2-8 µM (14). In the study of Hegazy et al., premyrsinanes named Euphosantianane A–D isolated from Euphorbia sanctae-catharinae with 3, 5, 7, 13, 15, 17-hexahydroxy-14-oxo-premyrsinane core structure showed moderate cytotoxicities with IC50 values of 20 to 75 µM against colon (Caco-2) and lung (A549) cancer cells (15). In a review done by Durán-Peña et al., premyrsinane biological activities were compared with other macrocyclic diterpenoids having the gem-dimethylcyclopropane subunit, including tiglianes, lathyranes, ingenanes, casbanes, jatropholanes, and premyrsinanes (16). They suggested that the gem-dimethylcyclopropane ring does not change in the different biosynthetic pathways, implying its essential role in the ecology of Euphorbia species (16). Vasas et al. isolated three 3, 5, 7, 14, 15-pentahydroxy-13(17)-epoxypremyrsinane derivatives and one 3, 5, 7, 14, 15, 17-hexahydroxy-13(17)-hemiacetal premyrsinane compound from E. falcata with moderate cytotoxicity against HeLa, A431, and MCF-7 cancer cells (17, 18). They also showed a good synergy effect with doxorubicin at 20 μM and the modulation of multidrug resistance in an MDR mouse lymphoma cell line. Among them, premyrsinanes with penta or hexa esters showed the most activity, and the only one with free hydroxy groups at 5, 7, and 15 positions were inactive (17).
5.1. Conclusions
Aceton: dichloromethane extract of E. aleppica was submitted to isolation by column chromatography, Sephadex LH-20, and normal-phase recycle HPLC. We isolated two rare diterpenes from premyrsinane types. The inhibitory effect of compounds 1 and 2 against the growth of breast cancer cells was evaluated with an MTT assay. Compound 1 showed moderate cytotoxicity. Compound 2 differed from compound 1 in the 17-O-acetylated hemiacetal ring, and the 14-O-acetyl group exhibited a potent cytotoxic effect in a dose-dependent manner against MDA-MB231 MCF-7 cancer cells. These results suggest other premyrsinane derivatives similar to compound 2 with 17-O-acetylated hemiacetal group and 14-O-acetyl group for extensive studies of the mechanism of anti-cancer effects, especially against breast cancer cells.