1. Background
Diabetes mellitus is a disease that occurs due to impaired insulin secretion or effect. As a result of the defect in carbohydrate, fat, and protein metabolism, blood sugar rises, and damage and dysfunctions develop in various organs in the long term (1). Insulin resistance occurs in type 2 diabetes and is associated with various disorders, such as obesity, hypertension, and hyperlipidemia (2). The inhibition of enzymes that metabolize carbohydrates is one of the treatment approaches for diabetes mellitus. Alpha-amylase, one of these enzymes, enables the breakdown of complex carbohydrates into smaller oligosaccharides; however, the α-glucosidase enzyme causes these oligosaccharides to break down into sugars with even smaller molecules. The inhibition of these enzymes prevents blood sugar from rising.
Diabetes mellitus also causes disorders in lipid metabolism. For this reason, it is important that these molecules act on enzymes that play a role in lipid metabolism in discovering candidate molecules that affect diabetes complications. Lipase plays a role in the breakdown of lipids and acts in their digestion and transport (3). Pancreatic cholesterol esterase is a serine hydrolase enzyme that plays an important role in the absorption of dietary cholesterol into the blood. Cholesterol esterase inhibition plays a role in the treatment of hypercholesterolemia and atherosclerosis by reducing the bioavailability of dietary cholesterol (4). It might be possible to treat these diseases or alleviate their complications by inhibiting such key enzymes that play a role in the pathophysiology of diabetes, obesity, and hyperlipidemia. Medicinal plants are important sources for discovering effective and safe new natural drug molecules in the treatment of diabetes mellitus.
Pistacia vera L. is a tree belonging to the Anacardiaceae family. The fruits and seeds of P. vera L. are traditionally used for skin conditions, such as abdominal ailments, abscesses, bruises, itching and sores, chest ailments, gynecological ailments, amenorrhea, dysentery, rheumatism, and trauma (5). The seeds inside the ripe fruit are edible and used in desserts, meats, sauces, and cakes. The fruits are known by the name “pistachio”. Since it is widely consumed as roasted, it is also grown in different countries, such as Turkey, Iran, the USA, the West of Asia, and the Middle East (6). In 2017, it was reported that pistachio production was 1,115,066 tons in 770,861 ha in the world, and the export rate of pistachio reached approximately 2.811 billion dollars (7). It is also interesting that although the fruits and seeds of the plant are widely consumed as food, its leaves have no economic or commercial importance in the field of food and medicine. The literature has shown that the leaves of P. terebinthus L. ssp. palaestina (Boiss.) Engler in Anatolia and P. atlantica Desf. in Jordan are traditionally used in folk medicine against diabetes (8, 9).
With the above-mentioned background in mind, the present study aimed to investigate the in vitro antidiabetic potential of P. vera leaves, on which no antidiabetic activity study has been conducted to date. For this purpose, the aqueous-alcoholic extract of P. vera leaves and its subextracts (i.e., petroleum ether [PE], chloroform [CHCl3], ethyl acetate [EtOAc], and n-butanol) were tested against enzymes involved in diabetes mellitus (i.e., α-glucosidase and α-amylase), obesity (i.e., pancreatic lipase), and hypercholesterolemia (i.e., cholesterol esterase). In addition, this study determined antioxidant activities (i.e., total antioxidant capacity, 2,2-diphenyl-1-picrylhydrazyl [DPPH] radical scavenging activity, metal chelating activity, and ferric-reducing capacity) and total flavonoid and phenolic contents of the leaf extract. The phytochemical profile of the aqueous-alcoholic extract of the leaves and the most active subextract were elucidated by tandem liquid chromatography quadrupole time-of-flight mass spectrometry (LC-QTOF-MS). The amount of 1,2,3,4,6-penta-O-galloyl-D-glucopyranose (PGG), which was determined to be the major compound in both the aqueous-alcoholic extract and the most active subextract, was measured by reversed-phase high-performance liquid chromatography (RP-HPLC).
2. Methods
2.1. Plant Material
The leaves of the P. vera were collected from Tillo, Siirt, Turkey, in July 2014 (Alt. 1100 m). The plant material was identified by M. Ufuk Özbek (Department of Biology, Gazi University, Ankara, Turkey). Herbarium materials were stored in the Herbarium of Faculty of Pharmacy, Gazi University (GUEF 3437).
2.2. Extraction of Plant Material
The leaves were dried in the shade at room temperature and ground. The dried leaves (190 g) were extracted with 3 L 80% ethanol for 24 hours, twice at room temperature. The filtrates were combined and concentrated using a rotary evaporator at 45°C (80% ethanol extract: 31.38 % w/w dry plant). The extract (45 g) was suspended in distilled water, and then fractionation was carried out using solvents with increasing polarity (PE, CHCl3, EtOAc, and n-butanol/saturated with water [n-BuOH]). All the subextracts were evaporated under reduced pressure at a temperature not exceeding 45°C. After this fractionation process, the remaining aqueous (R-H2O) phase was dried in a lyophilizer at -80°C. The yields of the five subextracts were calculated (PE: 15.43 w/w, CHCl3: 1.262 w/w, EtOAc: 16.407 w/w, n-BuOH: 13.02 w/w, and R-H2O: 43.794 w/w).
2.3. Total Phenolic Content of Crude Extract
The total phenolic content of the crude extract was determined by adding 10% (w/v) Folin-Ciocalteu reagent and Na2CO3 solution to the extract. After waiting 30 minutes in the dark, the absorbance of the mixture was read at 735 nm. The total phenol content of the extract was expressed in terms of gallic acid equivalent (GAE) mg/g of the extract (10).
2.4. Total Flavonoid Content of Crude Extract
The total flavonoid content of the crude extract was determined by adding 1 M sodium acetate and 10% (w/v) AlCl3 solutions to the extract. After waiting 30 minutes at room temperature, the absorbance of the mixture was read at 415 nm. The total flavonoid content of the extract was expressed in terms of quercetin equivalent (QE) mg/g of the extract (11).
2.5. Antioxidant Activities
2.5.1. DPPH Radical Scavenging Activity
In this study, 1 mM DPPH was added to the samples and left in the dark at room temperature for 30 minutes. The absorbance of the samples was read at 520 nm. Ascorbic acid was used as the reference compound (12).
2.5.2. Metal Chelating Activity
In this study, 2 mM FeCl2 solution was added to the samples and incubated for 5 minutes at room temperature. Then, 5 mM ferrozine solution was added. After 10 minutes, the absorbance of the samples was measured at 562 nm. Ethylenediaminetetraacetic acid (EDTA) was used as the reference compound (13).
2.5.3. Ferric-Reducing Antioxidant Power
In a 0.1 mol/L sodium phosphate buffer (pH = 7.2) 1% (w/v), potassium ferricyanide solution was added to the samples and incubated at 37ºC for 60 minutes. After incubation, 10% (w/v) trichloroacetic acid was added, and the absorbance was measured at 700 nm. After the measurement, 0.1% FeCl3 solution was added, and the measurement was made again; the difference between the two absorbance values was calculated. The QE was used as the reference compound (14).
2.5.4. Total Antioxidant Capacity
Molybdate reagent solution was mixed with the samples in the test tubes. The tubes were incubated at 90°C for 90 minutes. Then, the tubes were cooled immediately to room temperature, and the absorbance was measured at 695 nm. The results were expressed as ascorbic acid equivalent (AAE) (15). The equation of calibration was y = 1.5828x + 0.1354, r² = 0.9961
2.6. Enzyme Inhibitory Activities
2.6.1. Alpha-Glucosidase Inhibitory Activity
Alpha-glucosidase (Sigma Co., St. Louis, USA) Type IV prepared in 0.5 M phosphate buffer (pH 6.5) was incubated with the samples for 15 minutes, and the p-nitrophenyl-α-D-glucopyranoside solution (20 mM) was added as a substrate to the mixtures. After 35 minutes, the absorbance of the formed p-nitrophenol was read at 405 nm. The incubations were conducted at 37°C. Acarbose (Bayer) was used as the reference compound (16).
2.6.2. Alpha-Amylase Inhibitory Activity
Alpha-amylase (Sigma-Aldrich, Zwijndrecht, Netherlands) Type I (4 U/mL) was incubated with the samples for 5 minutes, and the starch solution (0.5% w/v, in phosphate buffer pH 6.9) was added as a substrate to the mixtures. The incubations were conducted at 37°C. After 3 minutes, 3,5-dinitrosalicylic acid solution (96 mM) prepared in 5.31 M sodium potassium tartrate (in 2 M NaOH) was added as a color reagent, and keep the mixtures were at 85°C for 40 minutes. The tubes were immediately cooled, and the absorbance of the mixtures was read at 540 nm. The formed maltose with the degradation of the starch was calculated using the maltose calibration equation
2.6.3. Pancreatic Lipase Inhibitory Activity
Pancreatic lipase (Sigma Co., St. Louis, USA) Type II prepared in a solution (pH 6.8) containing 10 mM 4-morpholinepropanesulfonic acid and 1 mM EDTA was incubated with the samples in a Tris (hydroxymethyl) aminomethane hydrochloride (Tris-HCl) buffer (pH 7.0) containing 100 mM Tris-HCl and 5 mM CaCl2 for 15 minutes. A substrate solution containing p-nitrophenyl butyrate (10 mM) in acetonitrile was added to the mixture. After 30 minutes, the absorbance of the formed p-nitrophenol was read at 405 nm. The incubations were conducted at 37°C. Orlistat (F. Hoffmann-La Roche AG, Basel, Switzerland) was used as the reference compound (18).
2.6.4. Cholesterol Esterase Inhibitory Activity
Cholesterol esterase (Sigma-Aldrich, Zwijndrecht, Netherlands) from the porcine pancreas was dissolved in 100 mM phosphate buffer (pH = 7) containing 100 mM NaCl. Furthermore, 12 mM taurocholic acid and substrate (p-nitrophenyl butyrate) solution were added to the samples in the phosphate buffer, respectively. The mixture was incubated for 5 minutes at 25ºC. Then, the enzyme was added, and the differences in the absorbance were determined using kinetic measurement at 405 nm for 6 minutes. Simvastatin was used as the reference compound (19).
2.7. Characterization of Crude Extract by LC-QTOF-MS
The analyses were carried out on Agilent 1260 series HPLC system and Agilent 6550 iFunnel High-Resolution Mass Spectrometer device connected to this system (Agilent Technologies, Inc., CA, USA). The analyses were made in the negative mode, and MS (mass spectrometry) system was utilized in the double spray Agilent Jet Stream Electrospray ionization technique. The MS operating mode is the 2 GHz extended dynamic range. Agilent MassHunter Software B06.00 and Metlin Metabolite database were used for data evaluation and analysis. For the separation, TC-C18 column (170 Å, 4.6 mm × 150 mm × 5 µm) (Agilent technologies 6410, USA) was used. The column temperature was 30°C; the injection volume was 5.00 µL; the flow rate was 0.650 mL/minute; the analysis time was 38 minutes. The mobile phase was set using solvent A (10 mM ammonia acetate) and solvent B (acetonitrile). The flow program was as follows:
0 minute 4% B, to 10 minutes 10% B, to 22 minutes 30% B, to 24 minutes 70% B, to 25 minutes 90% B, to 29 minutes 90% B, to 29.10 minutes 4% B, to 30 minutes 4% B.
UV: 280 nm (20 Hz); Ionization mode: Negative; Drying gas flow, nitrogen: 14 L/minutes; Drying gas temperature: 200°C; Nebulizer: 40 psi Sheath gas flow, nitrogen: 11 L/ minutes; Sheath gas temperature: 350°C; Nozzle voltage: 1000 V; Capillary voltage: 1400 V; Mass reading range: 40 - 1700 amu; Reference ions: 966.0007, 980.01637.
2.8. PGG Analysis in Crude Extract and EtOAc Subextract by RP-HPLC
The PGG determined by RP-HPLC in the crude extract and EtOAc subextract was isolated from Rhus coriaria by Gok et al. 2020 (20). The extract was prepared in 25% (v/v) acetonitrile solution at a concentration of 1 mg/mL and was transferred to vials by filtering through membrane filters. The PGG was also prepared in a 25% aqueous acetonitrile solution as a standard. The HP Agilent 1260 series LC System and ACE 5 C18 (5 μm, 150 mm × 4.6 mm) column were used. The column temperature was set as 25°C. The mobile phase was set using Solvent A (acetonitrile: H2O: formic acid [50:50:0.5]) and Solvent B (H2O: formic acid [100:0.5]). The gradient system was used for the separation of the peaks. The flow program was as follows:
0 minute 5% A, to 10 minutes 15% A, to 17 minutes 15% A, to 22 minutes 20% A, to 32 minutes 30% A, to 50 minutes 100% A, to 53 minutes 100% A.
The analysis time was set at 53 minutes. The flow rate was 0.8 mL/minute, and the injection volume was 20 µL. The calibration equation and correlation coefficient determined for PGG were obtained (y = 43.936x + 4.7902, r2 = 0.9998).
2.9. Statistical Analysis
Statistical analyses were conducted using the one-way analysis of variance and Dunnett’s Multiple Comparison Test. A P-value less than 0.05 was considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001).
3. Results
3.1. Total Flavonoid and Phenolic Contents of Crude Extract
The total flavonoid content of the aqueous-alcoholic extract was obtained as 33.48±2.07 mg/g QE. The equation of calibration of the total flavonoid content was
3.2. Antioxidant Activity
The DPPH scavenging activity of the aqueous-alcoholic extract of P. vera leaf showed as much activity as the reference compound ascorbic acid at all tested concentrations. The DPPH radical scavenging activity of the crude extract was slightly increased at low concentrations. It was concluded that the metal chelating capacity of the extract was significantly lower than EDTA at all concentrations. On the other hand, the increased absorption values in the ferric-reducing power assay indicated that the tested compounds had a high reducing ability. It was observed that the extract (3.928 - 3.915) had the same absorbance values as quercetin (3.940 - 3.941), especially at concentrations of 1 and 2 mg/mL. Again, the same extract gave a high absorbance value (2.961 ± 0.050) at 0.5 mg/mL concentration (Table 1). In addition, the total antioxidant capacity of the extract was determined as 97.68 ± 2.68 mg/g AAE.
| Samples Concentration (µg/mL) | DPPH Radical Scavenging Activity (Mean% ± Standard Deviation) | Metal Chelating Capacity (Mean% ± Standard Deviation) | Ferric-Reducing Power (Absorbance ± Standard Deviation) |
|---|---|---|---|
| Pistacia vera extract | |||
| 250 | 90.51 ± 0.40*** | 6.54 ± 1.26ns | 1.597 ± 0.090*** |
| 500 | 90.34 ± 0.77*** | 13.51 ± 1.27*** | 2.961 ± 0.050*** |
| 1000 | 88.93 ± 1.38*** | 22.76 ± 3.02*** | 3.928 ± 0.000*** |
| 2000 | 87.44 ± 0.55*** | 34.51 ± 1.34*** | 3.915 ± 0.000*** |
| References | |||
| 250 | 91.61 ± 0.15 a*** | 99.69 ± 0.18 b*** | 3.774 ± 0.170 c*** |
| 500 | 90.34 ± 0.77 a*** | 99.88 ± 0.36 b*** | 3.934 ± 0.000 c*** |
| 1000 | 91.35 ± 0.20 a*** | 100.03 ± 0.24 b*** | 3.940 ± 0.000 c*** |
| 2000 | 91.17 ± 0.57 a*** | 100.00 ± 0.13 b*** | 3.941 ± 0.000 c*** |
Abbreviations: DPPH, 2,2-diphenyl-1-picrylhydrazyl; ns, not significant.
a Ascorbic acid
b EDTA
c Quercetin
3.3. Enzyme Inhibitory Activities
This study evaluated the α-amylase, α-glucosidase, pancreatic lipase, and cholesterol esterase inhibitory activities of the aqueous-alcoholic extract of P. vera leaf. The crude extract specifically inhibited α-amylase and α-glucosidase with the half-maximal inhibitory concentration (IC50) values of 7.74 ± 0.72 and 11.08 ± 3.96 µg/mL, respectively; however, the reference compound acarbose inhibited these enzymes with IC50 values of 1.35 ± 0.10 and 0.95 ± 0.27 µg/mL, respectively. The crude extract (168.43 ± 26.10 µg/mL) displayed a very weak inhibitory effect on the pancreatic lipase enzyme, compared to orlistat (4.09 ± 1.08 µg/mL), and no effect on the cholesterol esterase enzyme. The subextracts obtained (i.e., PE, CHCl3, EtOAc, n-BuOH, and R-H2O) by activity-directed fractionation were also tested due to the strong inhibitory activity observed on the α-amylase and α-glucosidase of the crude extract. Especially when the EtOAc subextract was compared to the crude extract, it was determined that it caused stronger inhibition of α-amylase and α-glucosidase with IC50 values of 7.31 ± 1.36 µg/mL and 7.69 ± 0.05 µg/mL, respectively (Figure 1). Additionally, none of the subextracts showed significant inhibitory effects on pancreatic lipase and cholesterol esterase enzymes.
3.4. LC-QTOF-MS Analysis of Crude Extract
Seven organic acid derivative compounds were identified by LC-QTOF-MS in the aqueous-alcoholic extract of P. vera leaves. Gallic acid m/z 169.0151 [M-H]-, gentisic acid-O-hexoside m/z 315.1096 [M-H]-, methyl gallate m/z 183.0316 [M-H]-, ethyl gallate m/z 197.0462 [M-H]-, methyl digallate m/z 335.0416 [M-H]-, PGG m/z 939.1113 [M-H]-, and ethyl 2,4-dihydroxy-3-((3,4,5-trihydroxybenzoyl)oxy)benzoate m/z 349.0573 [M-H]- were tentatively annotated and identified using HRMS (high-resolution mass spectrometry) data and (MS/MS) (mass fragmentation) data and compared to fragmentation fingerprints given in the literature (20-25).
The presence of eight flavonoids in the same extract was tentatively annotated and identified using HRMS data and MS/MS data and compared to fragmentation fingerprints given in the literature. The predicted compounds and molecular weights were Gallocatechin m/z 305.0681 [M-H]-, myricetin 3-(6''-galloylhexoside) m/z 631.0958 [M-H]-, myricetin-O-rutinoside m/z 625.1418 [M-H]-, myricetin 3-O-hexoside m/z 625.1418 [M-H]-, myricetin 3-O-glucuronide m/z 493.0638 [M-H]-, quercetin 3-O-(6''-galloyl)-hexoside m/z 615.1012 [M-H]-, myricetin-3-O-α-pentoside m/z 449.0734 [M-H]-, and quercetin-3-O-glucuronide m/z 477.0690 [M-H]- (21, 24, 26-28). Table 2 shows detailed information about the data of the molecules and fragments.
| No. | TR [min] | [M-H]-exp [m/z] | [M-H]-calcd [m/z] | Δ [ppm] | Molecular Formula | MS/MS (-) [m/z] | Identification | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 7.769 | 169.0151 | 169.0142 | 5.05 | C7H6O5 | 125.0252 | Gallic acid | (24) |
| 2 | 12.794 | 305.0681 | 305.0667 | 4.67 | C15H14O7 | 261.0780; 219.0674; 125.0252 | Gallocatechin | (26) |
| 3 | 13.196 | 315.1096 | 315.0722 | 118.70 | C13H16O9 | 153.0564; 109.0302 | Gentisic acid-O-hexoside | (24, 25) |
| 4 | 17.886 | 183.0316 | 183.0299 | 8.21 | C8H8O5 | 124.0177; 78.0120 | Methyl gallate | (24) |
| 5 | 20.901 | 631.0958 | 631.0941 | 2.74 | C28H24O17 | 479.0850; 457.0785; 316.0243; 167.0358 | Myricetin 3-(6''-galloylhexoside) | (26) |
| 6 | 21.454 | 625.1418 | 625.1410 | 1.28 | C27H30O17 | 316.0236 | Myricetin-O-rutinoside | (24) |
| 7 | 21.789 | 479.0847 | 479.0831 | 3.34 | C21H20O13 | 316.0237; 271.0255; 178.9994 | Myricetin 3-O-hexoside | (26) |
| 8 | 22.057 | 493.0638 | 493.0624 | 3.04 | C21H18O14 | 317.0312; 178.9992 | Myricetin 3-O-glucuronide | (21) |
| 9 | 22.459 | 615.1012 | 615.0992 | 3.41 | C28H24O16 | 463.0895; 300.0287; 169.0149 | Quercetin 3-O-(6''-galloyl)-hexoside | (27) |
| 10 | 22.710 | 197.0462 | 197.0455 | 3.55 | C9H10O5 | 169.0148; 124.0170; 78.0115 | Ethyl gallate | (22) |
| 11 | 22.978 | 449.0734 | 449.0726 | 2.00 | C20H18O12 | 316.0236; 271.0255; 197.0465 | Myricetin-3-O-α- Pentoside | (28) |
| 12 | 23.246 | 335.0416 | 335.0409 | 2.39 | C15H12O9 | 183.0310; 124.0169 | Methyl digallate | (23) |
| 13 | 23.648 | 939.1113 | 939.1109 | 0.43 | C41H32O26 | 769.0909; 617.0792; 169.0148 | Pentagalloyl glucose | (20) |
| 14 | 23.849 | 477.0690 | 477.0675 | 3.35 | C21H18O13 | 301.0365 | Quercetin-3-O- glucuronide | (24) |
| 15 | 27.132 | 349.0573 | 349.0565 | 2.29 | C16H14O9 | 197.0465; 124.0166 | Ethyl 2,4-dihydroxy-3-(3,4,5-trihydroxybenzoyl) oxybenzoate | (23) |
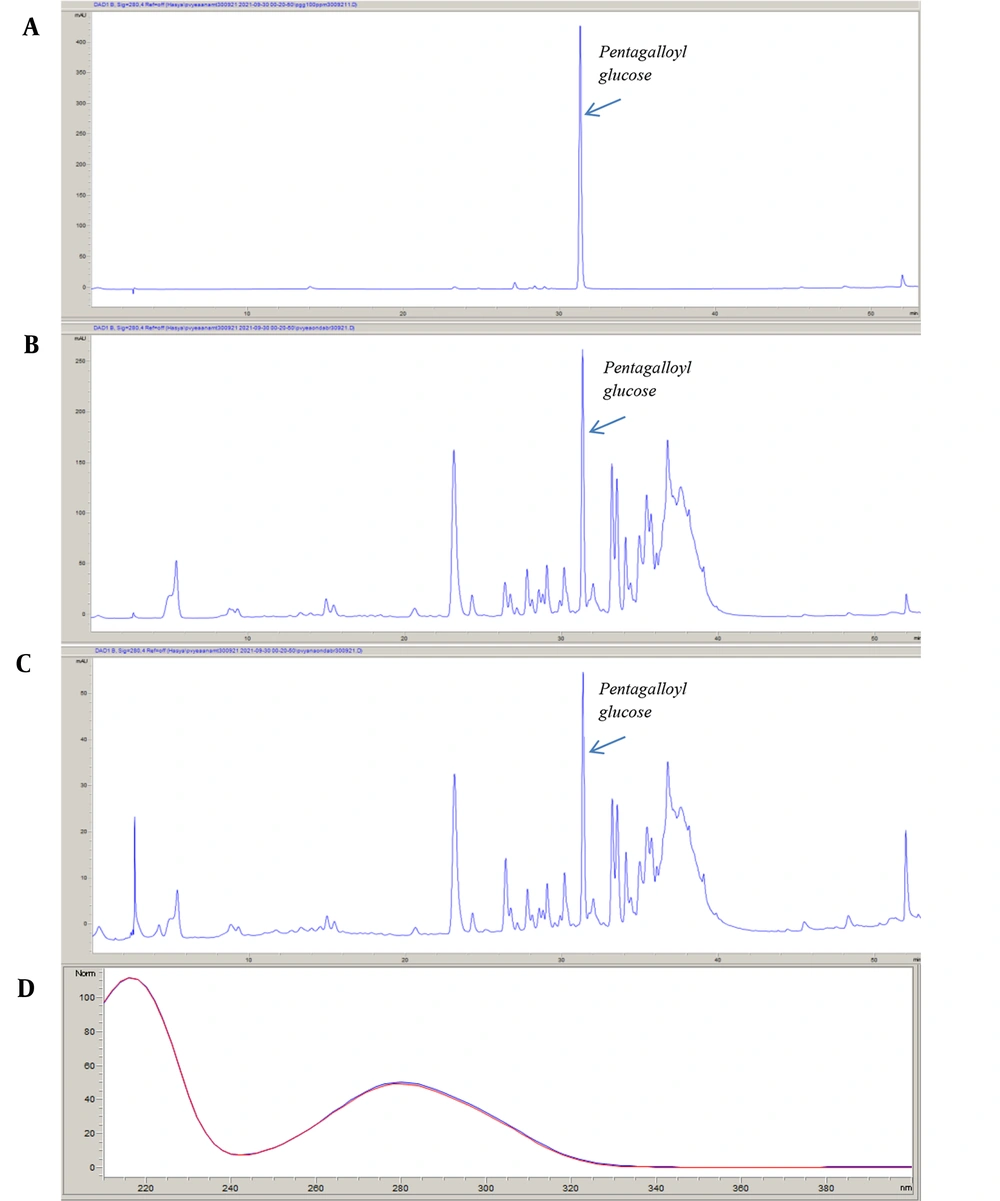
3.5. RP-HPLC Analysis of PGG in Crude Extract and EtOAc Subextract
The PGG, which has an inhibitory effect on α-glucosidase, α-amylase, and pancreatic lipase enzymes isolated from R. coriaria leaves by Gok et al. (20), was also detected as a result of LC-QTOF-MS analysis of P. vera crude extract. For this reason, PGG was used to standardize both the crude extract and the EtOAc subextract, which is the most effective subextract. The retention time of PGG was 31.329 minutes. The amount of PGG was observed to be higher in the EtOAc subextract (5.794 ± 0.027 g/100 g) than in the crude extract (1.295 ± 0.001 g/100 g). Figure 2 illustrates the chromatograms of standard PGG, P. vera crude extract, and EtOAc subextract.
4. Discussion
The α-amylase, α-glucosidase, pancreatic lipase, and cholesterol esterase enzyme inhibitory activities of P. vera leaves were investigated in the present study. As a result, the hydroalcoholic extract of the leaves showed remarkable α-amylase (77.36 ± 7.13 µg/mL IC50) and α-glucosidase (110.84 ± 39.64 µg/mL IC50) activities. The EtOAc subextract obtained from the crude extract by liquid fractionation also showed the strong inhibition of α-amylase and α-glucosidase enzymes. These enzymes play a role in carbohydrate digestion and cause the carbohydrates consumed to be broken down into glucose. By inhibiting these enzymes, carbohydrate digestion can be slowed down, thereby reducing glucose absorption and elevating blood glucose levels. This is considered an important treatment approach, especially in type II diabetes. On the other hand, since a metabolic disease, such as diabetes mellitus, might be a cause of oxidative stress in the body, it is important that P. vera extract has antioxidant activity.
When the in vitro, in vivo, and clinical antidiabetic activity studies on P. vera were examined, it was concluded that these studies mostly evaluated the fruits of the plant in terms of this activity. Lalegani et al. (29) evaluated the α-amylase and α-glucosidase activities of polyphenol-rich extract from pistachio green hull (PGH). The PGH inhibited α-amylase from human saliva, porcine pancreas, and Bacillus sp. with 109.05 ± 0.01, 174.14 ± 0.06, and 305.25 ± 0.04 IC50 values (µg GAE/mL), respectively. The PGH also inhibited α-glucosidase from Saccharomyces cerevisiae with a 6.10 ± 0.02 IC50 value (µg GAE/mL).
In addition, the antioxidant activity of PGH was assessed by DPPH and ABTS (2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt) radical scavenging methods. The PGH showed DPPH and ABTS radical scavenging activities with IC50 values of 44.08 ± 0.01 and 139.84 ± 0.04 µg/mL, respectively. Moreover, considering the results of both activity experiments, the antioxidant activity of PGH was higher than the reference compound Trolox. On the other hand, the phenolic compounds of PGH were analyzed by RP-HPLC; phloroglucinol (65.40 ±2.33 mg/g), gallic acid (5.22 ± 0.21 mg/g), and vanillic acid (2.30 ± 0.02 mg/g) were determined as major compounds. In addition to the major compounds, protocatechuic acid, 4-hydroxybenzoic acid, catechin, eriodictyol-7-O-glucoside, and naringin were detected in PGH (29).
A randomized, crossover, controlled nutrition study on 30 adults with type II diabetes examined the effects of daily pistachio consumption on lipid/lipoprotein profile, glycemic control, inflammation markers, and endothelial function. There was no significant change in body weight, body mass index, waist-to-hip ratio, blood pressure, total cholesterol, triglycerides, low-density lipoprotein (LDL), high-density lipoprotein (HDL), and fasting blood sugar levels at the end of 12 weeks in patients who consumed 42 g/day and 70 g/day pistachios. At the end of the 12th week, the blood glucose level of the patients in the second group (70 g/day) was observed to be lower than at the beginning; nevertheless, the triglyceride level of the patients in the first group (42 g/day) decreased significantly (P = 0.018) (30).
In a double-blind, randomized, placebo-controlled, crossover study to examine the effects of P. vera fruits on blood glucose levels in 48 patients with type 2 diabetes, a group of patients consumed 25 g of these fruits twice daily for 12 weeks. At the end of the 12 weeks, the patients had an 8-week washout period. Then, the other group of patients consumed the same amount of P. vera fruits for 12 weeks. At the end of the 12 weeks, a decrease was observed in hemoglobin A1c (0.4%) and fasting blood glucose (16 mg/dL), compared to those of the control group (P < 0.001) (31).
In a randomized, crossover, controlled nutrition study of 30 adults (40 - 74 years) with type 2 diabetes, the participants consumed a pistachio or pistachio-free diet for 4 weeks. After the 2-week washout period, the patients changed groups and continued to be fed with pistachio or pistachio-free diet for 4 more weeks. Following the pistachio diet, total cholesterol and triglyceride levels were observed to be significantly reduced, compared to those of the control diet; nonetheless, no difference was observed in fasting glucose and insulin levels (32).
In vivo antidiabetic activity studies were also conducted on other Pistacia species. The antidiabetic activities of P. lentiscus leaf and fruit extracts were analyzed in vivo on a streptozotocin (STZ)-induced diabetes mellitus model in rats. Pistacia lentiscus leaf extract was shown to be more active than the fruit extract. The leaf extract decreased serum glucose level at 125 mg/kg dose as nearly as the reference drug glibenclamide (33).
Behmanesh et al. aimed to evaluate the role of the hexane extract (200 mg/kg) of P. atlantica seeds in protecting against ovarian damage in an STZ-induced diabetes model in rats. In the aforementioned study, which continued for 4 weeks, the animals were given the extract every day. At the end of the experiment, blood sugar levels, oxidative stress parameters, and histological ovarian structure were evaluated. Blood glucose, malondialdehyde levels, and atretic follicle count were elevated in diabetic rats; catalase, superoxide dismutase levels, and the number of corpus lutea were significantly decreased. On the other hand, it was determined that the aforementioned values returned to normal or decreased in animals treated with P. atlantica extract and glibenclamide (34).
The α-amylase and α-glucosidase enzyme inhibitory activities of EtOAc subextract obtained from 70% acetone extract of P. atlantica leaves were examined in a study by using the Partial Least Squares regression and fingerprints correlation optimized warping. Based on the results of the study, only α-amylase was inhibited by glucogallin, quinic acid, and galloyl quinic acid in the leaves, thereby concluding that methyl gallate and tetragalloyl glucose inhibited only α-glucosidase; nevertheless, both α-amylase and α-glucosidase were inhibited by gallic acid, gentisic acid, and digalloyl quinic acid (35).
In a study examining the antidiabetic activity of P. terebinthus extract in STZ-induced diabetic rats, it was determined that the extract reduced blood sugar, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, lactate dehydrogenase, glucose, total triglyceride, total cholesterol, HDL, and LDL levels (36). An in vitro study investigated the α-amylase, α-glucosidase, and pancreatic lipase inhibitory activities of aqueous extracts of P. lentiscus and P. terebinthus fruits and leaves. It was determined that the most active extract against pancreatic lipase was P. lentiscus leaf extract (6.1 ± 0.2 µg/mL IC50). Pistacia terebinthus leaf, P. terebinthus, and P. lentiscus fruit extracts inhibited the same enzyme system with IC50 values of 9.0 ± 0.4, 125.2 ± 12.1, and 230.7 ± 38.4 µg/mL, respectively. Pistacia lentiscus and P. terebinthus leaf and fruit aqueous extracts were observed to inhibit α-amylase and α-glucosidase enzymes with IC50 values ranging from 65.3 ± 7.4 µg/mL to 1.4 ± 0.2 mg/mL, respectively. The findings of the study exerted that Sardinian Pistacia species can be used as functional foods and nutraceuticals that modulate carbohydrate and lipid digestion and absorption as potential sources to prevent obesity and diabetes mellitus (37).
Studies carried out on the chemical profile of P. vera have mostly focused on nuts, kernels, and oleoresin. Gallic acid, catechin, epicatechin, eriodictyol-7-O-glucoside, genistein-7-O-glucoside, naringenin-7-O-neohesperidoside, quercetin-3-O-rutinoside, genistein, eriodyctiol, daidzein, quercetin, naringenin, luteolin, kaempferol, apigenin, cyanidin-3-O-galactoside, and cyanidin-3-O-glucoside were detected by the HPLC analysis of pistachio seeds and skins. Among the quantified compounds, quercetin-3-O-rutinoside was observed to be the main compound in seeds (98.08 ± 1.54 µg/g) and cyanidin-3-O-galactoside in skins (5865.12 ± 362.45 µg/g fresh weight) (38).
It has been determined by scientific studies that PGG has strong α-amylase and α-glucosidase inhibitory activities. In a study performed on 14 ellagitannins, it was determined that the most active compounds against α-glucosidase were α-pentagalloyl glucose (1.2 ± 0.3 µM IC50) and PGG (1.4 ± 0.2 µM IC50), and both of these compounds (32.9 ± 1.8 and 17.2 ± 1.6 µM IC50, respectively) showed high inhibitory activity against α-amylase (39). On the other hand, Gok et al. determined that pancreatic lipase was inhibited by 43.22% (200 μg/mL) and 48.68% (10 ng/mL) by PGG and orlistat, respectively, used as a reference (20). Since this compound has high enzyme inhibitory activity and is the main compound in both crude extract and EtOAc subextract as a result of RP-HPLC analysis, the standardization process was performed on this compound.
It has been reported in previous scientific studies that 7 (i.e., ethyl gallate, gallic acid, gallocatechin, methyl gallate, myricetin 3-[6''-galloylhexoside], quercetin 3-O-(6''-galloyl)-hexoside, and quercetin-3-O-glucuronide) of 15 compounds detected in the crude extract by LC-QTOF-MS have α-glucosidase enzyme inhibitory effect, and one (gallic acid) of them has α-amylase enzyme inhibitory effect (20, 40-46). The compounds, including gallic acid, methyl gallate, ethyl gallate, gallocatechin, myricetin 3-(6''-galloylhexoside), quercetin 3-O-(6''-galloyl)-hexoside, and quercetin-3-O-glucuronide, that were observed to have α-amylase and α-glucosidase inhibitory effects in previous studies were detected by LC-QTOF-MS in P. vera leaf extract. Apart from the aforementioned compounds, the presence of six phenolic compounds, namely gentisic acid-O-hexoside, methyl digallate, ethyl 2,4-dihydroxy-3-((3,4,5-trihydroxybenzoyl)oxy)benzoate, myricetin-O-rutinoside, myricetin 3-O-hexoside, myricetin 3-O-glucuronide, and myricetin-3-O-α-pentoside, in P. vera leaves was detected for the first time in this study, and they might be responsible for the activity through synergistic interactions.
Flavonoids and phenolic acids are secondary metabolites commonly detected in plants with numerous biological activities, such as antioxidant, antidiabetic, anticancer, antibacterial, cardioprotective, and anti-inflammatory activities (47). Different parts of P. vera contain various secondary metabolites, such as tannins, phenolic acids, flavonoids, and anthocyanins (48). In the present study, the high total phenol content of P. vera leaves was responsible for the antioxidant activity.
4.1. Conclusions
In this study, the in vitro antidiabetic, antihypercholesterolemic, and antiobesity effects of P. vera leaves were evaluated for the first time. Pistacia vera leaf hydroalcoholic extract and EtOAc subextract showed potent α-amylase and α-glucosidase enzyme inhibitory activities. It has also been supported by literature information that especially seven phenolic compounds identified in the plant by LC-QTOF-MS have inhibitory activities on these enzymes. In the light of the aforementioned data, it was concluded that the plant can be used as a standardized medicinal herbal product for the prevention and treatment of diabetes or as a source for the isolation of bioactive compounds. It also suggested that the antioxidant effect of the plant might be beneficial for diabetes complications. Future studies should be performed on the evaluation of the leaves of the P. vera in terms of metabolic diseases with in vivo experimental models and the isolation of their active compounds.
![Enzyme inhibitory activities (µg/mL half-maximal inhibitory concentration [IC<sub>50</sub>]) of crude extract and subextracts of <i>Pistacia vera.</i> Enzyme inhibitory activities (µg/mL half-maximal inhibitory concentration [IC<sub>50</sub>]) of crude extract and subextracts of <i>Pistacia vera.</i>](https://services.brieflands.com/cdn/serve/3170b/eb038d71306c54991ffacfd89414fb791debe1a2/ijpr-127033-i001-F1-preview.webp)