1. Background
The healing procedure is the same in all wounds occurring, consequently in the corruption of skin integrity. Wound healing includes processes that restore the barrier function and mechanical integrity of the skin. This complex dynamic procedure comprises hemostasis, inflammatory, angiogenesis, and remodeling steps (1). Hemostasis is the first response to the wound healing process. Inflammatory, angiogenesis, and remodeling phases require intercellular interactions and the contribution of released components, such as extracellular matrix (ECM) (2-4). Neutrophils and macrophages are essential in the inflammatory phase and responsible for pathogen removal at the wound site (3). Matrix metalloproteinases (MMPs) are crucial in processes such as remodeling, angiogenesis, and wound healing. As a result of increased MMP-9 activity in the wound area, the wound healing process may be inactivated, and stabilizing these factors is likely to raise wound healing capacity (4).
Wounds pose a significant socioeconomic burden on society due to their high prevalence and recurrence (1). The medical cost of wound curation is high and continues to increase quickly. Therefore, there is a requirement for a better biological and clinical understanding of the mechanisms that support wound repair and for finding effective therapeutic agents to alleviate the financial burden on patients and the country (5).
Due to the complexity of the content of plants, researchers have focused on investigating the effect of a single active ingredient of plants in recent years to avoid these complications (6-8). TRP, which is present in indigo plants, is an indole quinazoline alkaloid. The natural product tryptanthrin (indolo[2,1-b]quinazoline-6,12-dione) is a weakly basic alkaloid. This brilliant yellow compound comprises a quinazoline ring fused to an indole moiety within carbonyl groups in the 6- and 12-positions. The name tryptanthrin is derived from the production of this compound by the yeast Candida lipolytica when grown in an L-tryptophan-containing medium. TRP derivatives, which are native and chemically synthesized, have been found and bear a varied functional group at the sixth position of a common indolo[2,1-b]quinazoline core. It was first isolated from the culture of yeast C. lipolytica and later from the Chinese medicinal plant Strobilanthes cusia Kuntze. It can also be gained from other herbal species, such as Isatis, Clanthe, Wrightia, and Couroupota (9). Many studies have revealed that TRP has many biological and pharmacological activities, including antimicrobial, anti-inflammatory, anti-allergic, anti-cancer, anti-protozoal, and anti-allergic effects (8-13). The excisional wound (EW) model utilized in the present research is the most used wound model, and it is easy to register the time-dependent closure rate of the wound by forming an open wound (14).
2. Objectives
This study aimed to evaluate the effect of the TRP compound, which has antimicrobial, anti-inflammatory, and antioxidant properties, on the EW model in mice. Furthermore, we tried to explain the possible effects of vascular endothelial growth factor (VEGF) and MMP-9 on wound healing.
3. Methods
3.1. Experimental Procedure
3.1.1. Excision Wound Model
We utilized the excision wound model improved by Morton and Malone (15). The excision wound was made on the dorsal part of the mice 1 cm away from the spine and 5 cm from the ears under anesthesia with a combination of ketamine and xylazine (20/10 mg/kg). An excision wound model was formed by removing a circular part of two wound, nearly 4 mm in diameter and 2 mm in depth, per animal, using Kai Sterile Dermal Biopsy Punch (Japan). The animals were divided into five groups healthy, EW, EW + 1 mg/kg, EW + 2.5 mg/kg, and EW + 5 mg/kg (16). Three doses of 1, 2.5, and 5 mg/kg of TRP (CAS: 13220-57-0, 97%, MW:248.24, BLDpharm, UK) were administrated topically to the wound part every day for 14 days, and the test was terminated when complete healing was observed in the wound parts on the 14th day. To assess the therapeutic effectiveness of TRP, the wound closure rate was assessed on days 0, 3, 7, and 14 by applying a caliper (150 mm, Mesem®, China).
3.1.2. Evaluation of Wound Closure
Wound closure was assessed using a caliper on days 0, 3, 7, and 14, and the wound closure percentile was figured out with the following formula:
3.2. Subjects and Experimental Groups
A total of 90 BALB-C female mice aged 6 - 8 weeks were utilized in the present investigation. Mice were held in a checked room (22 ± 1°C, relative humidity of 50% - 60% on a 12-hour light/dark cycle) and were fed tap water and pellet food. Mice were randomly allocated into five groups, with 18 mice in each group. Among the animals in each group, 6, 6, and 14 were utilized for analysis on days 3, 7, and 14, respectively.
3.3. Vascular Endothelial Growth Factor and Matrix Metalloproteinase-9 Analysis in Wound Explants
In order to examine the influence of TRP on VEGF and MMP-9 levels in the wound area, VEGF and MMP-9 levels in tissue explants from mice were analyzed by enzyme-linked immunosorbent assay (ELISA). Vascular endothelial growth factor (catalog No: E0114Mo, Bioassay Technology Laboratory, China) and MMP-9 (catalog No: E0277Mo, Bioassay Technology Laboratory, China) in wound explants were taken on the 3rd, 7th, and 14th days and were analyzed using the ELISA kit. The results were compared with the values obtained from healthy controls.
3.4. Vascular Endothelial Growth Factor and Matrix Metalloproteinase-9 Analysis in Serum Samples
On the last day of the experiment, intracardiac blood samples were taken from each mouse by cardiac puncture into gel vacuum tubes under anesthesia. After centrifugation at 3000 rpm for 20 min, VEGF and MMP-9 levels were analyzed by ELISA. Vascular endothelial growth factor (catalog No: E0114Mo, Bioassay Technology Laboratory, China) and MMP-9 (catalog No: E0114Mo, Bioassay Technology Laboratory, China) in blood samples taken on day 14 were measured by ELISA. The results were compared with the values obtained from healthy controls.
3.5. Histopathological Evaluation
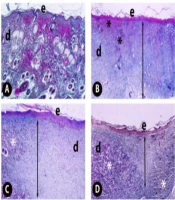
Skin specimens were fixed in 10% neutral buffered formaldehyde for 72 h at room temperature for histopathological assessments, and specimens were deposited in paraffin blocks. Sections of approximately 5 μm were cut from paraffin blocks with a Leica RM2125RT microtome (Leica Microsystems, Wetzlar, Germany). Crossman’s modified triple staining technique was applied for all groups, and the samples were investigated under a light microscope (Nikon Eclipse i50, Tokyo, Japan), and photographs were taken. Histopathological scoring was performed for each group using a previously described method (17) to demonstrate epidermal and dermal regeneration. All sections were evaluated in terms of epithelial proliferation, formation, granulation tissue thickness, inflammatory cells, and angiogenesis in the wound area. For this purpose, scoring was performed by evaluating 20 random areas per section from each sample with a × 40 objective lens.
3.6. Statistical Analysis
The data were assessed statistically utilizing SPSS software 22.00 (IBM, USA). It was conducted utilizing a Tukey test, which comprised one-way ANOVA and posthoc multiple comparative assays.
4. Results
4.1. Wound Closure Rates
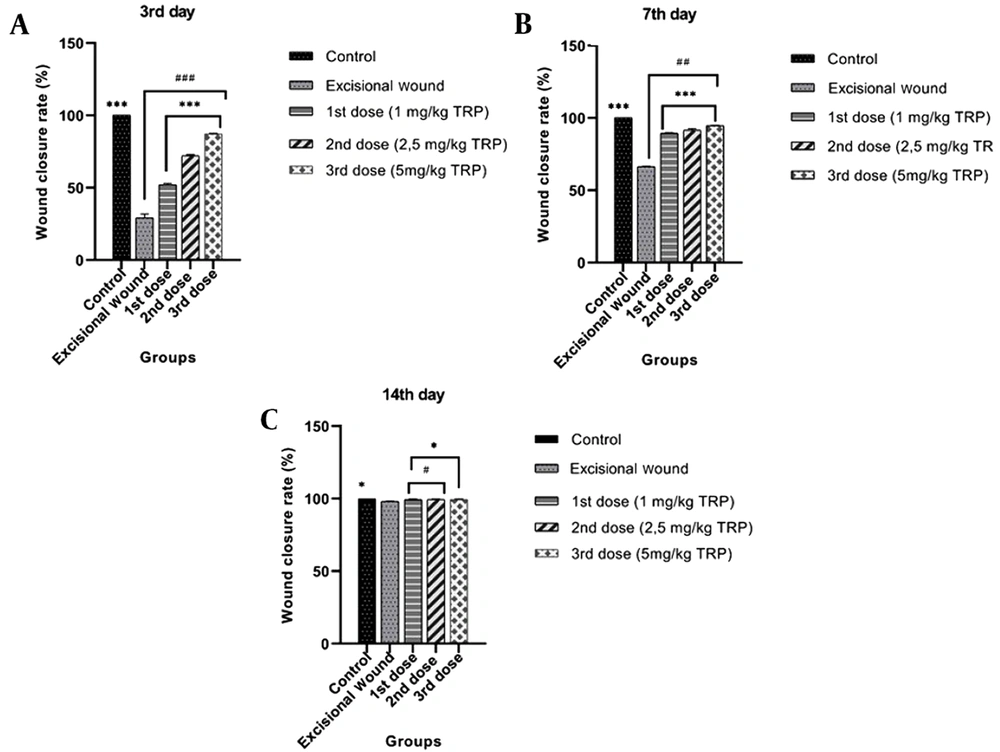
In order to examine the effect of the TRP compound on excisional wound healing in mice, the TRP compound was applied topically for 14 days after creating a wound model, and wound closure rates on days 0, 3, 7, and 14 were measured using a digital caliper. Our findings revealed that the wound closure percentages of the TRP molecule on the third day were 52.12%, 72.20%, and 87.22%, and significant improvements were found in the wound areas with these rates. On the seventh day, the percentage of wound closure was found to be 94% in the groups which received the third dose of TRP. When the wound closure rates were investigated on day 3 (Figure 1A), day 7 (Figure 1B), and day 14 (Figure 1C), the wound model was formed. It was detected that the wound areas were closed due to the enhanced dose of TRP compound.
4.2. Vascular Endothelial Growth Factor of Wound Explants
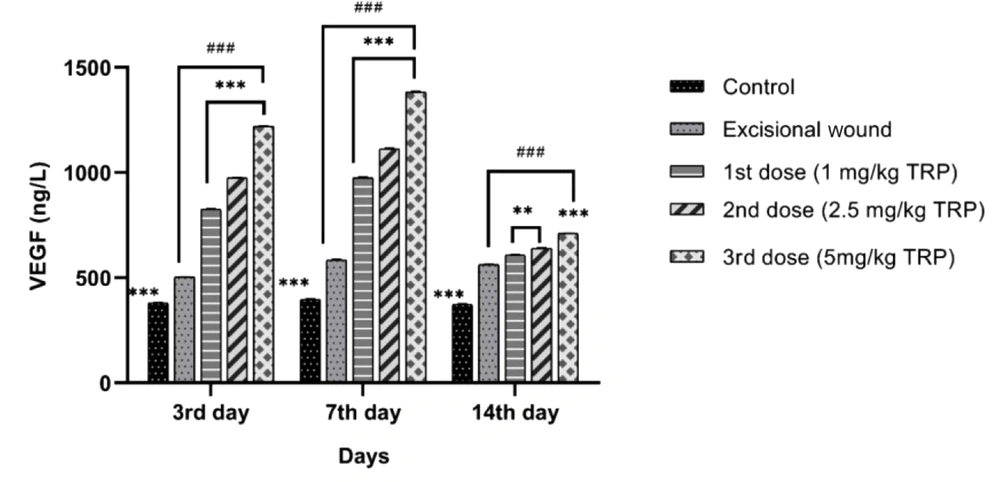
On days 3, 7, and 14, VEGF levels were higher in the excisional wound group compared to the healthy controls group. A dose-dependent enhancement was detected in the groups treated with excisional wound + TRP compared to the excisional wound group with a statistically significant difference (P < 0.001). The VEFG level on days 3, 7, and 14 (Figure 2) indicated that the rates for the excisional wound group increased gradually on days 3 and 7, depending on the increasing dose of TRP, and decreased on day 14.
4.3. Matrix Metalloproteinase-9 of Wound Explants
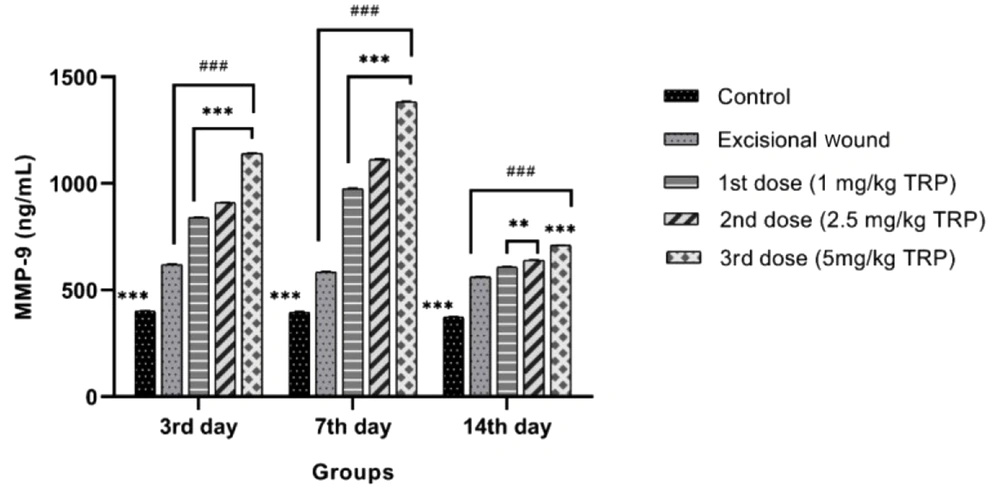
On the 3rd, seventh, and 14th days, MMP-9 levels were higher in the excisional wound group than in the healthy control group. It was determined that in the TRP groups, it increased dose-dependently compared to the excisional wound group with a statistically significant difference (P < 0.001). The MMP-9 level on days 3, 7, and 14 (Figure 3) revealed that the rates for the excisional wound group rose gradually on days 3 and 7 depending on the increasing dose of TRP and declined on day 14 with the completion of healing.
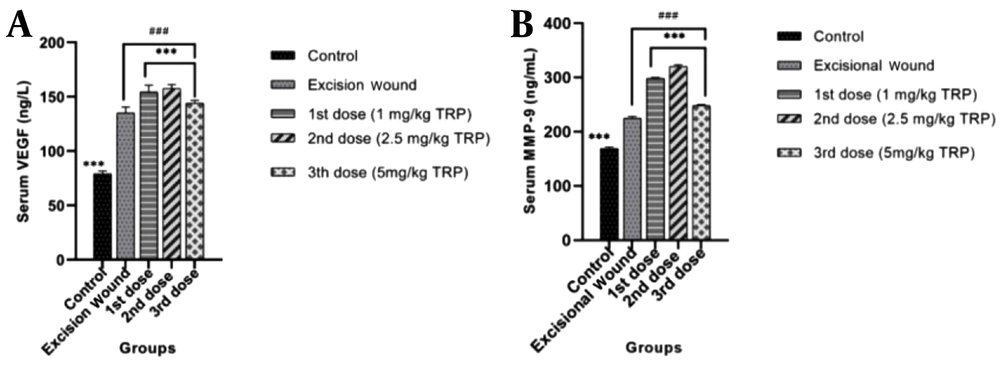
4.4. Vascular Endothelial Growth Factor and Matrix Metalloproteinase-9 of Serum Samples
The VEGF (Figure 4A) and MMP-9 (Figure 4B) levels in intracardiac blood samples taken on day 14 are shown in Figure 4. Vascular endothelial growth factor and MMP-9 levels, which have important roles in wound healing processes, increased in the groups where the 1st dose (1 mg/kg) and 2nd dose (2.5 mg/kg) of TRP were applied, and the 3rd dose of TRP (5mg/kg) was found to be decreased in the groups to which it was applied. The decline in serum VEGF and MMP-9 levels in the groups which received the third dose of TRP indicated that the wound healing process was completed.
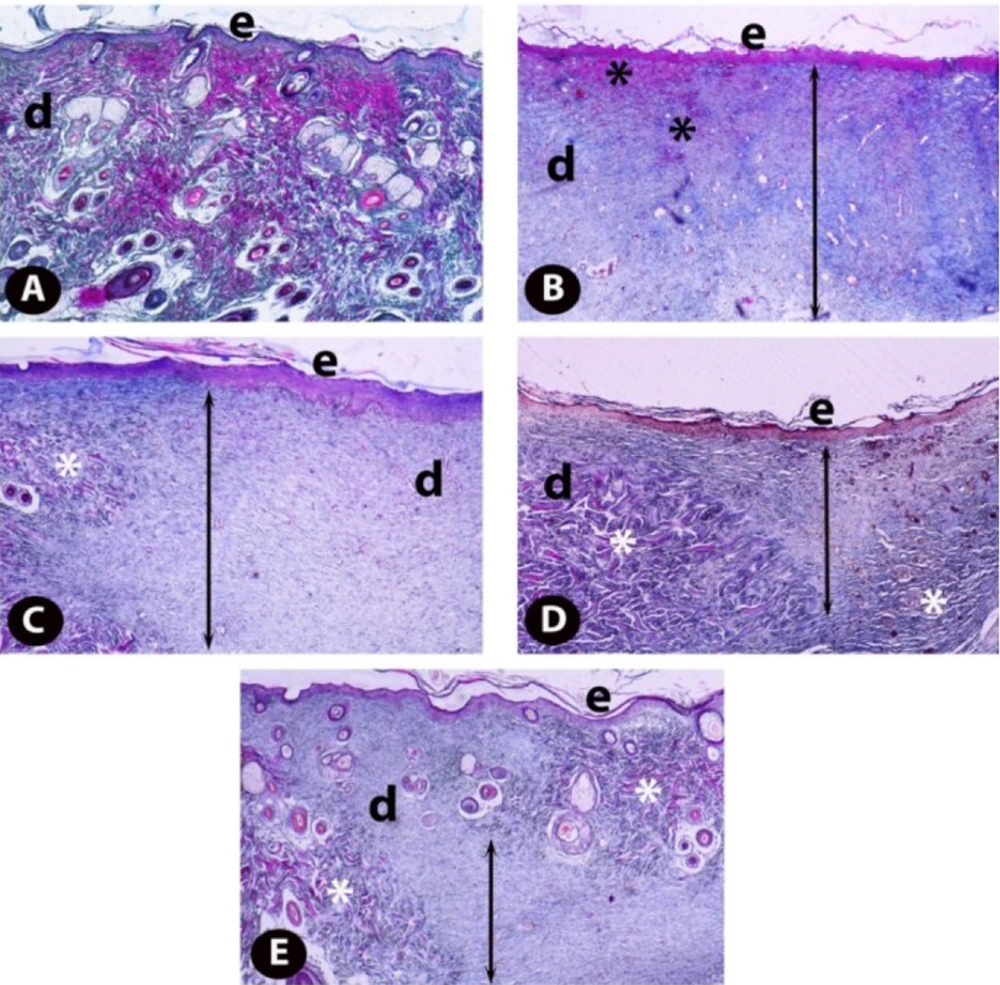
4.5. Histopathological Findings
The examination of the sections stained with Crossman’s modified triple staining technique under the light microscope revealed that the skin samples of healthy controls (Figure 5A) had a normal histological structure. Comparing the skin samples of the excisional wound group with the healthy controls showed that epidermis regeneration in the wound area was insufficient, and the epidermal development was incomplete. In addition, dermal regeneration was also very low, and edema and congestion areas rich in inflammatory cells and thick granulation tissue in the dermis of the wound area were remarkable (Figure 5B). When TRP-administered groups were examined, it was found that epidermis regeneration increased in the 1 mg/kg TRP group compared to the wound group, and the edema and congestion areas in the dermal area began to decrease, while there was still a thick granulation tissue (Figure 5C). In the excisional wound groups, 5 and 2.5 mg/kg groups had the highest healing levels. Regeneration was completed in the epidermis of the excision area of the rats and the newly formed blood vessels in the dermis layer of the wound area (Figure 5D). Collagen fiber bundles were synthesized and started to organize, and connective tissue proliferation was striking. Furthermore, granulation tissue was considerably reduced (Figure 5E). Scores of histopathological findings obtained from the groups are presented in Table 1.
| Control | EW | EW + 1st Dose b | EW + 2nd Dose c | EW + 3rd Dose d | |
|---|---|---|---|---|---|
| Reepithelization | 3.75 ± 0.44 | 1.85 ± 0.49 | 2.10 ± 0.45 | 2.75 ± 0.44 | 3 ± 0.56 |
| Granulation tissue thickness and collagen matrix | 1.70 ± 0.47 | 2.90 ± 0.45 | 2.45 ± 0.51 | 2 ± 0.56 | 1.85 ± 0.75 |
| Inflammatory cells | 3.90 ± 0.31 | 0.90 ± 0.55 | 1.35+0.49 | 2.30 ± 0.47 | 3.40 ± 0.50 |
| Angiogenesis | 3.65 ± 0.49 | 0.85 ± 0.59 | 1.70 ± 0.47 | 2.25 ± 0.44 | 3.20 ± 0.70 |
Abbreviation: EW, excisional wound
a Values are expressed as mean ± Standard deviation.
b 1 mg/kg TRP
c 2.5 mg/kg TRP
d 5 mg/kg TRP
5. Discussion
In this paper, we evaluated the effect of the TRP compound, which has antimicrobial, anti-inflammatory, and antioxidant properties (18), on the excisional wound model in mice. According to our results, the wound closure percentages of TRP molecule, especially on the third day, were 52.12%, 72.20%, and 87.22%, with significant improvements in the wound areas compared to the healthy controls group. Examination of VEGF and MMP-9, which have important roles in wound healing, indicated that the wound healing of TRP-administered groups improved on the third and seventh days as expected and declined on the 14th day in line with complete healing.
For centuries, medicinal herbs have been utilized in traditional medicine and have been recorded to heal wounds by minimizing pain and scarring (6). The failure of wound healing can lead to severe complications and even death (19). The therapeutic agent utilized in wound healing must possess antimicrobial, cell proliferation, and antioxidant properties. The literature review has pointed out that TRP possesses antimicrobial, antioxidant, and anti-inflammatory properties. Therefore, this compound was selected for wound healing (9). Investigations have revealed that TRP has numerous biological and pharmacological activities, such as antimicrobial, anti-cancer, anti-inflammatory, anti-allergic, and anti-protozoal effects (8-12). Moreover, compounds coated with TRP (13, 20-22) have been shown to have anti-inflammatory, antimicrobial, wound healing, anti-tumor, anti-allergic, and other biological activities. Another study demonstrated that the indigo compound promoted wound closure in human keratinocyte HaCaT cells (23).
Our study results indicated that the TRP molecule caused essential improvements such as wound closure rate, especially on day 3, and the recovery rate was 94% in the groups that received the third dose of TRP on day 7. However, healing in the excisional wound group was delayed until the 14th day. The relevant studies (24-28) revealed that healing in the wound areas started from day three, and the healing rates were very high on day 7. The findings of the current study were consistent with other studies. It was also observed in line with other studies that TRP completely healed the wound area over time.
Vascular endothelial growth factor is an important biomarker in wound healing (29) and increases vascular permeability, allowing the flow of inflammatory cells to the wound area. Moreover, it facilitates tissue repair by preventing the migration and proliferation of pre-existing endothelial cells. Decreased VEGF level is associated with defective healing (30). In research (31), VEGF was high in cells that migrated for repair after wound formation, and these levels remained high in the course of granulation tissue formation until epidermal closure was complete. In our study, it was determined that VEGF levels rose on days 3 and 7 and decreased on day 14, depending on the increasing dose in the groups treated with TRP compared to the excisional wound groups. The high levels of VEGF in the groups treated with TRP indicated the positive effect of TRP on angiogenesis.
Matrix metalloproteinases have a considerable role in accelerating wound healing via stimulating angiogenesis. Studies have shown the inhibition of cell proliferation and delayed wound closure in MMP-9 knockout mice. Matrix metalloproteinase-9 contributes to wound repair by modulating VEGF release (32). In other studies (31-33), high levels of MMP-9 were associated with late recovery. The current study revealed that MMP-9 levels increased on the third and seventh days and declined on the 14th day, depending on the increasing dose in the groups treated with TRP compared to the excisional wound group. The latter finding indicated that the TRP molecule positively affects the wound healing rate.
Blood vessels, fibroblasts, collagen fibers, and angiogenesis were assessed to evaluate the inflammatory phase. In addition, collagen regulation plays an important role in the remodeling phase and thus in the subsequent scar formation phase of wound closure. It was found that histopathological analyses performed on the 14th day of wound healing increased re-epithelialization and collagen content in the wound area (34). In our study, the highest level of healing was found in the 5 mg/kg TRP group compared to the excisional wound group, and it was closest to the histological structure in the healthy control group. In the sections belonging to this group, it was observed that epidermis regeneration was at a very high level, the granulation tissue in the dermis considerably reduced, and the collagen fiber bundles began to cover a large part of the dermis.
5.1. Conclusions
After applying TRP in the excisional wound model, it has been proven macroscopically and microscopically that wound healing reached the maximum level on the third day and completely healed on the 14th day in a dose-dependent manner compared to the healthy controls group. We showed that this improvement after TRP application might be due to the levels of MMP-9 and VEGF, which induce neovascularization, which is very important in wound healing. Furthermore, we proved this improvement histopathologically. Therefore, it could be concluded that TRP has the potential to be used in threpeutic agent in wound healing due to its positive effects on wound healing. However, further clinical studies are required.