1. Context
Donepezil hydrochloride with the formula of C24H30ClNO3 and molecular weight of 416 Dalton is a second-generation acetylcholine esterase inhibitor (AChEI) that was first produced by the Eisai Company (1-3). This drug consists of a benzylpiperidine part bound to dimethoxy indanone via the methylene group (4). Donepezil binds selectively, reversibly, and non-competitively to the peripheral anionic site of acetylcholine esterase (AChE) (2, 5). It has a greater tendency to inhibit AChE than butyrylcholine esterase (BuChE), among other AChEIs, and some studies represented the clinical significance of this difference, especially in Alzheimer's disease (AD) (5, 6).
Donepezil has a relatively slow gastrointestinal absorption and takes 3 to 5 hours to reach peak plasma concentrations. Furthermore, the absorption of this drug does not interfere with food and, at the same time, has good bioavailability. Donepezil binds relatively well to serum proteins like albumin and is excreted more readily in the urine. This drug also has hepatic metabolism. Cytochrome P450 CYP2D6, CYP3A4, CYP3A5, and CYP2C9 are the enzymes involved in the metabolism of donepezil. However, CYP2D6 and CYP3A4 are thought to have the primary role in the metabolism of this drug in the liver (5, 7). On the other hand, this medicine has a half-life of 70 hours, reducing the number of tablets required during the day and improving patient compliance (5, 8). The steady-state concentration (Css) of this drug increases with dose so that it is about 30 ng/mL at the dose of 5 mg/day and about 60 ng/mL at 10 mg/day. However, the concentration of donepezil in the cerebrospinal fluid (CSF) is much lower than in plasma but is still dose-dependent (5, 8).
It also appears that donepezil has low drug interactions and good tolerability in side effects, especially at lower doses (5, 8). Common adverse effects of this drug include cholinergic side effects, such as nausea, vomiting, diarrhea, insomnia, anorexia, fatigue, muscle cramps, increased gastrointestinal secretions, and even effects on the vagus nerve and cardiac arrhythmias, which are dose-dependent (5, 9, 10).
Although donepezil was approved by the US Food and Drug Administration (FDA) to treat various stages of AD, and most studies have examined the effect of this drug on AD (5, 10), donepezil has some therapeutic potential in other dementia and non-dementia diseases such as improving movement and dementia in Parkinson's disease and positively affecting sleep disorders, psychiatric diseases, and even infectious diseases (11-14). Therefore, we aimed to review these effects in the present study, in addition to the role of this drug in treating AD. Hence, we surveyed these therapeutic potentials into two general topics: "donepezil and dementia" and "donepezil and non-dementia diseases."
2. Evidence Acquisition
This study aimed to review the studies on different therapeutic potentials of donepezil. The search keywords included donepezil hydrochloride, AD, memory impairment, dementia, cognitive impairment, vascular dementia, Parkinson's dementia, traumatic brain injury dementia, multiple sclerosis, neurologic disease, neuropsychiatry disease, Parkinson's disease, depression, mood disorders, antidepressant, schizophrenia, autism spectrum disorder, sleep disorders, immune system, infections, and COVID-19.
We reviewed all studies and articles in English in the national and international journals published between 2008 and 2022 in valid databases such as web of science, Google scholar, PubMed, Scopus, Cochrane, and science direct. Then, clinical trials, preclinical studies, case reports, cohorts, narrative reviews, systematic reviews, and meta-analyses related to the aims of the present study were included. Also, keywords were chosen based on the frequency and importance of studies in the initial search.
2.1. Donepezil and Dementia
2.1.1. Alzheimer's Disease and Donepezil
Alzheimer's is a progressive neurodegenerative disease affecting patients' cognitive and executive skills. It is one of the most frequent kinds of dementia and has the potential to cause disability and perhaps death. An estimated 57 million people worldwide suffered in 2019, which is expected to multiply by 2050. Therefore, this disease is a challenge to health care systems regarding cost burden and various consequences for individuals, families, and care systems (10, 15, 16). Therefore, donepezil is one of the drugs approved by the FDA to treat this disease in mild, moderate, and severe states, used both as monotherapy and in combination therapy with other drugs such as memantine. In addition to improving the symptoms of AD, this medicine has shown to be reasonably successful in slowing down the development of the disease, particularly in the early stages by the early prescription of the drug. It is, nevertheless, a reasonably safe medicine with a few adverse effects that are well tolerated (5, 10, 17, 18). Based on the evidence, it seems that different mechanisms, such as amyloid-beta (Aβ) plaque accumulation, tau pathology, glutamate excitotoxicity, oxidative stress, neuroinflammation, and vascular changes are involved in the pathophysiology of AD, which together lead to the neuronal and synaptic loss and atrophy in some areas such as the hippocampus and prefrontal cortex, responsible for cognitive, behavioral, and executive processes. As a result, studies show that donepezil may influence these pathways via various mechanisms, altering disease symptoms and progression (15, 18-20).
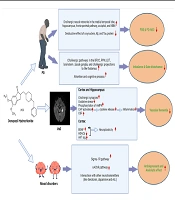
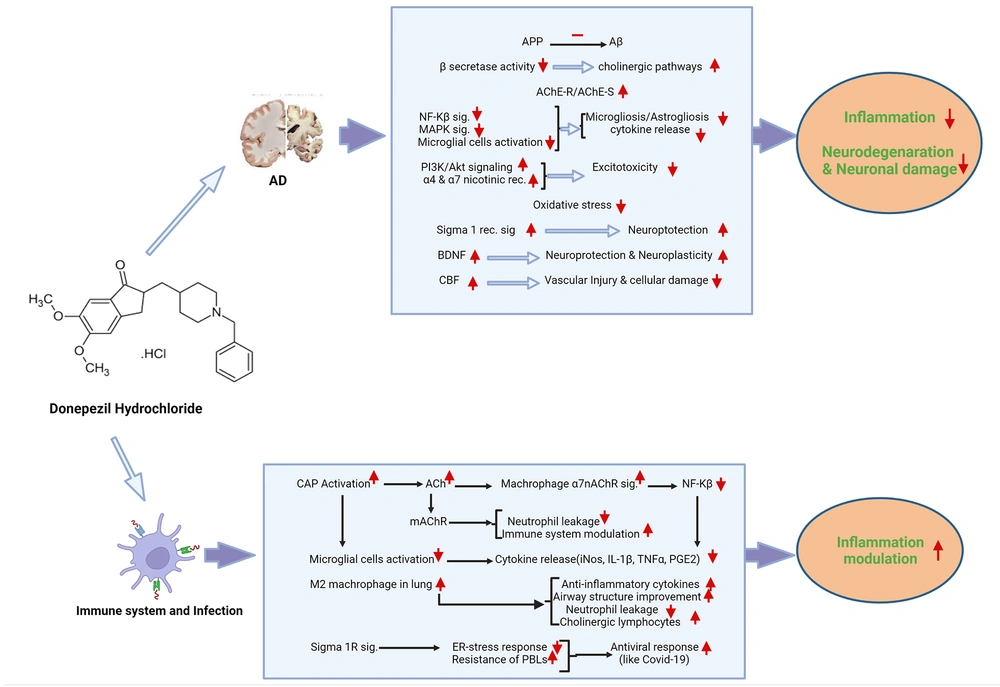
As known, one of the most important pathologies of AD is the accumulation of Aβ in the critical areas of the brain, such as the hippocampus. According to various preclinical and clinical studies, donepezil can reduce the accumulation and destructive effects of Aβ in the brain via different mechanisms, such as reducing the conversion of amyloid precursor protein (APP) to Aβ, increasing cholinergic pathways and synapses in the brain, followed by a decreased beta-secretase activity that is involved in Aβ production, and increasing the ratio of acetylcholinesterase read-through isoform (AChE-R), which has a protective effect on the neurons, to synaptic isoform (AChE-S), whose high expression leads to neuronal damage (Figure 1) (15, 17, 18).
Summary of donepezil effects on Alzheimer's disease and immune system. Abbreviations: AD, Alzheimer's disease; APP, amyloid precursor protein; Aβ, amyloid-beta; AChE-R, acetylcholinesterase read-through isoform; AChE-S, acetylcholinesterase synaptic isoform; NF-KB, nuclear factor-kappa B; MAPK, mitogen-activated protein kinase; Sig., signaling; PI3K/Akt pathway, phosphatidylinositol 3-kinases/protein kinase B pathway; Rec, receptor; BDNF, brain-derived neurotrophic factor; CBF, cerebral blood flow; CAP, cholinergic anti-inflammatory pathway; Ach, acetylcholine; α7nAChR, alpha 7 nicotinic acetylcholine receptor; mAChR, muscarinic acetylcholine receptor; iNOS, inducible nitric oxide synthase; IL-1β, interleukin-1β; TNFα, tumor necrosis factor alpha; PGE2, prostaglandin E2; Sigma 1R sig., sigma-1 receptor signaling; ER-stress, endoplasmic reticulum stress; PBLs, peripheral blood leukocytes.
Studies have shown that Aβ could activate microglial cells via the nuclear factor-kappa B (NF-kB) and mitogen-activated protein kinase (MAPK) signaling pathways, which, in turn, activate inflammatory pathways and secrete neurotoxins and cytokines, consequently leading to cell apoptosis and neurodegeneration processes in the CNS, especially in the hippocampal formation. Donepezil can inhibit the NF-kB and MAPK signaling pathways, as well as the activation of microglia cells. It can also reduce the production of cytokines and inflammatory factors, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), IL-6, nitric oxide (NO), and prostaglandin E2 (PGE2) in a dose-dependent way. It can also inhibit the expression of proinflammatory factor genes such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2).
On the other hand, donepezil may suppress the release of other neurotoxic factors from microglia, as well as the processes of microgliosis and astrogliosis, which worsen inflammation, and have protective effects on neurons and memory via these pathways (15, 18-20). Besides, one of the cell-destructive mechanisms in AD is glutamate toxicity and excitotoxicity process, which enhances the harmful effects of Aβ accumulation and tau pathology. Therefore, donepezil can play a protective role against these destructive processes by activating the Phosphatidylinositol 3-kinases/protein kinase B (PI3K-Akt) pathway and increasing α4 and α7 nicotinic receptors, which reduce the activation of N-methyl-D-aspartate (NMDA) receptors and apoptosis following calcium accumulation in the neurons (15, 18, 21, 22). Donepezil also protects the hippocampus and cerebral cortex from oxidative stress by inhibiting glutathione reduction and reducing inflammatory processes (15, 18, 23, 24). Donepezil may also affect the pathophysiology of AD via binding to the sigma-1 receptor, which is found in many areas of the brain, such as the CA1 region of the hippocampus, thalamus, cortex, cerebellum, and spinal cord. It is found on the membrane of the cell's endoplasmic reticulum. Moreover, it has a neuromodulatory, neuroprotective, neuroplasticity, and anti-apoptotic role. Therefore, donepezil, with the high binding capacity to this receptor, can play a protective role against pathologies and destructive mechanisms of AD, such as beta-amyloid accumulation and tau pathology (Figure 1) (15, 18, 25, 26).
Increased brain-derived neurotrophic factor (BDNF), which has a neuroplasticity impact on the cortex and hippocampus, is another protective mechanism of donepezil in AD. According to studies, donepezil can reduce ischemia-induced cellular damage and maintain the function of neurons in different areas of the brain, especially the medial temporal lobe (hippocampus and parahippocampus), cingulate gyrus, and prefrontal cortex, by regulating blood flow in cerebral arteries and reducing amyloid accumulation-induced microvascular damage (Figure 1) (15, 18).
Bond et al. concluded that donepezil was more cost-effective than other AChEIs, memantine, placebo, and high-quality supportive care in AD with mild to moderate severity in a systematic review and economic analysis (27). Furthermore, in a meta-analysis by Li et al., which analyzed the clinical trials of the efficacy and safety of common drugs used to treat AD, such as donepezil, galantamine, rivastigmine, and memantine, it was found that donepezil had significant efficacy in stabilizing or slowing down the reduction in cognitive and executive functions, behavioral indices, and global changes in AD patients with mild, moderate, and severe severity; it was also relatively safe in terms of tolerability and side effects (28). In another study, Kim et al. examined the effect of donepezil on the pathophysiology of AD in an animal model. They found that donepezil, both in vitro and in vivo, had a substantial modifying influence on disease-causing and exacerbating pathways, delaying disease progression and enhancing memory (19). Zhang and Gordon reviewed the effects of donepezil on treating AD in Chinese patients and showed that it was highly effective in treating mild to moderate AD and was probably influential in treating the severe type. It also reduced AD biomarkers, such as Aβ, tau protein, and hippocampal atrophy (29). However, according to a review study by Adlimoghaddam et al., donepezil can be effective in treating AD at different stages, although this effect is little (10). Furthermore, some studies have shown that donepezil has no significant effect on mild cognitive impairment and amnesia and could not prevent the progression of mild cognitive impairment to AD (30). It should be noted that based on various studies, the effectiveness of donepezil in AD treatment increases with increasing doses (5, 10, 17, 27). However, side effects are also more common at higher doses (Table 1) (10, 31).
| Type of Disease | Author | Type of Study | Dosage and Method of Administration | Number of Participants | Outcomes Summary |
|---|---|---|---|---|---|
| Donepezil and Non-Alzheimer's Dementia | |||||
| Parkinson's dementia | Wang et al. (32) | Systematic review and meta-analysis | 5 and 10 mg/daily, oral | Five trials examining DPZ | DPZ improved patients' daily functioning and cognitive symptoms. It was safe and could be helpful for MCI-PD, PDD, and LBD. |
| Vascular dementia | Kim et al. (33) | Meta-analysis | 5 and 10 mg/daily, oral | Five trials examined DPZ | AChEIs, including DPZ, significantly improved memory scale scores and maintained this improvement within 24 weeks through a stable pattern. They were well-tolerated. |
| Vascular dementia | Battle et al. (34) | Meta-analysis and network meta-analysis | 5 and 10 mg/daily, oral | Three trials studying DPZ (2,193 participants) | According to the meta-analysis, DPZ 5 and 10 mg had a negligible effect on cognitive improvement, although the side effects of the 10 mg dose were higher; However, according to the network meta-analysis, all AChEIs were more effective and safer than placebo. |
| Traumatic-brain injury dementia | Wheaton et al. (35) | Meta-analysis | 5 - 10 mg/day, oral | Six studies (DPZ/DPZ + physostigmine + lecithin) | Improvement of memory and attention impairment in the post-acute phase of TBI |
| Traumatic brain injury dementia | Campbell et al. (36) | Retrospective, longitudinal analysis | 5 - 10 mg/day, oral | 129 patients (55 patients in the DPZ group) | There was no significant difference between the two groups regarding cognitive factors such as processing speed, attention, and memory. |
| Donepezil and Non-dementia Diseases | |||||
| Parkinson's disease | Chung et al. (37) | Cross-over RCT | 5 - 10 mg/day, oral | 23 patients with PD with falls or near falls > 2 times per week | The number of falls per day in the DPZ group was almost half that of the placebo group. |
| Parkinson's disease | Chen et al. (12) | Meta-analysis | 5 and 10 mg/daily, oral | Three studies examining DPZ | AChEIs could not significantly improve gait and imbalance, although these drugs appeared to be relatively effective in regulating steps during a single activity. |
| Mood disorders | Reynolds et al. (38) | Clinical trial | 5 - 10 mg/day, oral | 130 patients with normal cognition or MCI | Improvement of executive function and memory; More recurrence of depression than the placebo group |
| Mood disorders | Fitzgerald et al. (39) | Animal study | 0.02 - 0.2 mg/kg, and 2 mg/kg, intraperitoneally injected | 160 mice | The antidepressant-like effect at lower doses and depressant-like effect at higher doses |
| Mood disorders | Hosseini et al. (40) | Clinical trial | 5 mg/day, oral | 73 patients aged 20 - 50 years old (37 patients in the DPZ group and the rest in the placebo group) | DPZ significantly improved cognitive impairment caused by SSRIs |
| Autism spectrum disorders | Gabis et al. (41) | Clinical trial | 2.5 - 5 mg/day, and DPZ 5 mg/day + Choline 350 mg/day for four weeks, oral | 60 autistic children and adolescents (5 - 16 years old) | Significant safety and efficacy in young children (younger than 10 years old); Less effective in adolescents with some significant side effects like increased irritability. |
| Autism spectrum disorders | Rossignol and Frye (42) | Systematic review | 2.5 - 30 mg/day, oral | Seven studies | Five studies showed improvement of many ASD symptoms (four of five case series and one of two RCTs). |
| Immune system and infectious disease | Abe et al. (43) | Retrospective cohort study | - | 25,602 hospitalized elderlies with pneumonia (578 patients taking DPZ for dementia) | DPZ acted as an independent factor in reducing hospitalized pneumonia patients' mortality. |
| Immune system and infectious disease | Sochocka et al. (44) | Experimental | 5 - 100 µg/mL, in vitro | PBLs from 30 blood samples (15 with resistant leukocytes to VSV and 15 with sensitive leukocytes) treated with DPZ or EGB-761 | 10 - 50 μg/mL DPZ increased resistance of human leukocytes and reduced activation of NF-κB. |
Summary of Studies Regarding the Various Therapeutic Potentials of Donepezil
According to studies and guidelines in treating mild to moderate AD, donepezil 5 mg per day is typically used for four to six weeks, after which it can be increased to 10 mg daily. The dose can be increased to 23 mg daily for patients with moderate to severe AD treated with donepezil 10 mg daily after at least three months (10, 31).
2.1.2. Parkinson's Dementia and Donepezil
Although Parkinson's disease (PD) is often considered a neurodegenerative motor disorder, it was shown to have a range of cognitive and memory impairments (32, 45). It seems that this cognitive disorder has a relatively high prevalence. Some studies have shown a 40% prevalence of mild cognitive disorder in patients in the early stages, which can increase the risk of dementia in the higher stages of the disease. Moreover, some studies indicated an 80% prevalence of dementia among patients with advanced stages of the disease. This condition is critical because of its negative consequences for patients and their families (45). Various types of cognitive impairments in Parkinson's disease may be manifested; however, it can be categorized into two categories: (1) Parkinson's disease with mild cognitive impairment (PD-MCI) that mainly affects the executive areas of the brain and working memory, such as frontal lobes and frontal-striatal pathways and is divided into two sub-categories: Single domain PD-MCI, which one cognitive domain impairment is detected without other cognitive domains damage in two abnormal cognitive test results, and multidomain PD-MCI, which more than one cognitive domain damage is detected in at least one abnormal test., and (2) Parkinson's disease dementia (PDD), a destructive cognitive impairment that can affect broad domains of cognition such as semantic verbal fluency, attention, and visual-spatial strength, originated from damage to the temporal lobe and other areas (45-47).
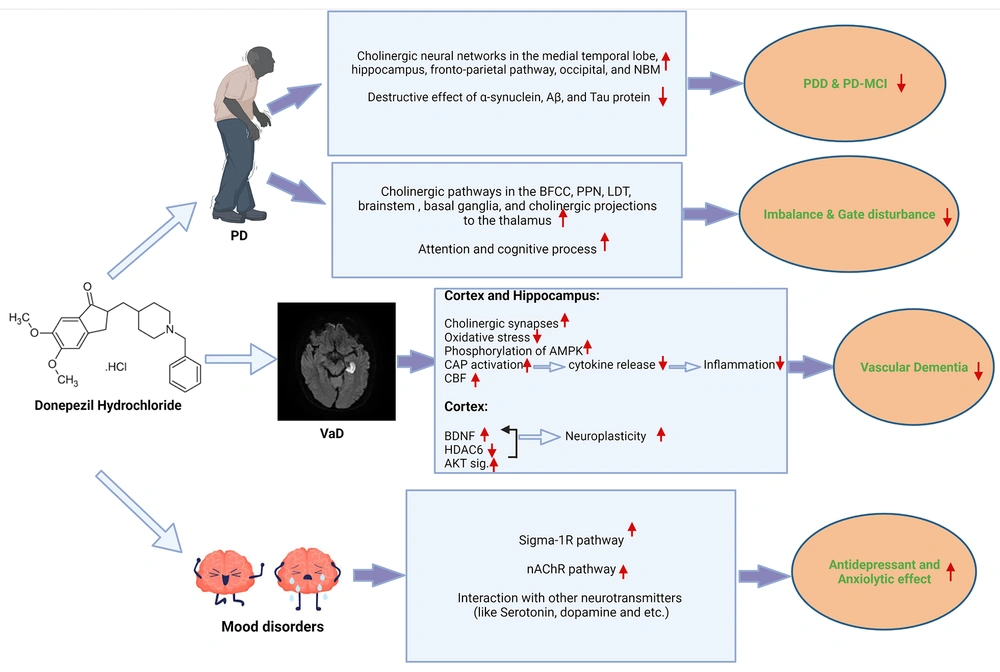
Various mechanisms have been proposed for the pathophysiology of various manifestations of cognitive impairment due to Parkinson's disease, such as the prominent role of dopaminergic and noradrenergic networks and receptors in areas of the brain related to executive function, including frontostriatal and mesocortical pathways, the prominent role of cholinergic neural networks in the process of attention, memory, executive function, and in areas such as the medial temporal lobe, especially the hippocampus, frontoparietal, occipital, and nucleus basalis of meynert (NBM), and the role of the noradrenergic system in many processes like concentration and executive function, such as the locus coeruleus and the frontoparietal pathway (11, 45, 48). Because of the importance of cholinergic neural pathways in the pathophysiology of PD-MCI and PDD, as well as the destruction of these pathways and innervations by mechanisms like alpha-synuclein, Aβ, and tau protein accumulation, AChEIs like donepezil and rivastigmine have been considered in many studies (Figure 2). Moreover, the results indicated the appropriate effectiveness of these drugs in various manifestations of cognitive impairment and dementia due to PD and improvement of patients' general cognition and executive function; in some studies, these effects were even more significant than the effects of these drugs in AD patients (13, 45, 48).
Summary of donepezil effects on Parkinson's disease dementia and motor impairments, vascular dementia, and mood disorders. Abbreviations: PD, Parkinson's disease; NBM, nucleus Basalis of Meynert; Aβ, amyloid-beta; PDD, Parkinson's disease dementia; PD-MCI, Parkinson's disease with mild cognitive impairment; BFCC, basal forebrain cholinergic complex; PPN, pedunculopontine nucleus; LDT, latero-dorsal tegmental nucleus; VaD, vascular dementia; AMPK, AMP-activated protein kinase; CAP, cholinergic anti-inflammatory pathway; CBF, cerebral blood flow; BDNF, brain-derived neurotrophic factor; HDAC6, histone deacetylase 6; AKT, protein kinase B; Sigma-1R, sigma-1 receptor; nAChR, nicotinic acetylcholine receptor.
In a placebo-controlled randomized, double-blind clinical trial study of donepezil efficacy and safety in PDD patients, performed by Dubois et al. with a relatively high sample size (550 participants) and doses of 5 and 10 mg donepezil daily, donepezil could be relatively effective with minimal side effects and well tolerability, improving cognition, general condition, and executive function of PDD patients (49). Wang et al. performed a systematic review and meta-analysis of clinical trials on the effectiveness and harmlessness of cholinesterase inhibitors and memantine in PDD, PD-MCI, and Lewy bodies dementia (LBD) before May 2013. This research revealed that donepezil could improve patients' daily functioning, cognitive symptoms, and overall effectiveness in clinicians' global impression of change. In terms of adverse effects, it is a safe medicine and may benefit PD-MCI, PDD, and LBD (32) (Table 1). Despite all the above results, due to different findings about the efficacy of this drug in Parkinson's dementia, donepezil has not been approved yet and is used off-label, and only rivastigmine has been approved among AChEIs (11, 13).
2.1.3. Vascular Dementia
Vascular cognitive impairment (VCI) is another cognitive disorder that ranges from mild to severe (34, 50). This entity occurs due to vascular impairment, especially in brain vessels (34, 51). Vascular dementia is one of the most common types of VCI and the second most common dementia after AD. Memory loss, executive dysfunction like decision-making and problem-solving, speech impairment and aphasia, visual impairment, behavioral and mood disorders like anxiety and depression, and even gait and balance impairments are common signs and symptoms of this dementia (34, 51, 52). Although no drug has been approved for treating this dementia, various studies were performed on cholinesterase inhibitors, including donepezil, which has been approved for treating AD (33, 34, 52, 53). Kim et al. meta-analyzed seven studies that investigated the effect of AChEIs on memory impairment after CVA and vascular dementia, finding that these drugs, including donepezil, significantly improved memory scales scores, such as mini-mental state examination (MMSE) and AD assessment scale-cognitive subscale (ADAS-cog) scores, and also maintained memory improvement within 24 weeks through a stable pattern compared to the placebo. These medications are also well tolerated and generally safe regarding adverse effects (33). Another meta-analysis of eight clinical studies by Battle et al. found that donepezil 5 mg might enhance cognition in individuals with vascular dementia or other cognitive vascular impairments, albeit the result was not clinically significant. It was also found that donepezil 10 mg daily was probably a little more than 5 mg effective in cognitive impairment but still not clinically significant. However, the side effects of donepezil 10 mg appeared to be slightly higher than those of 5 mg compared to the placebo. On the other hand, according to a network meta-analysis, all AChEIs were in a higher position than the placebo in terms of efficacy and a lower position in terms of side effects. It was also found that donepezil 10 mg/day ranked first in terms of benefits and third in terms of side effects among other AChEIs analyzed in this meta-analysis (including donepezil 5 mg/day, rivastigmine 3 to 12 mg/day, and galantamine 16 to 24 mg/day) (Table 1) (34).
According to studies, donepezil affects the pathophysiology of vascular dementia via various mechanisms. In a study by Jian et al., which examined the effect of donepezil on vascular dementia in rats experimentally subjected to bilateral obstruction of the common carotid artery, this drug could increase neuronal activity and prevent neurodegeneration due to vascular impairment through various mechanisms, such as increasing BDNF gene expression (an essential factor in the neuroplasticity and neuronal activity) directly and indirectly (by reducing nuclear translocation of histone deacetylase 6 (HDAC6), which is a regulatory factor in BDNF gene expression) in the cortex (regarding the critical role of the cortex in long-term memory), returning the density of dendritic synapses to near normal in the hippocampus and cortex, reducing oxidative stress due to free radicals, activating the AMP-activated protein kinase (AMPK) pathway in the hippocampus and cortex, and activating the protein kinase B (AKT) pathway in the cortex (52). Another study by Sharma and Singh which examined the effects of telmisartan and donepezil on vascular dementia due to hypertension induced by deoxycorticosterone acetate-salt in mice, achieved similar results and found that donepezil via mechanisms, such as activating the cholinergic anti-inflammatory pathway to inhibit the secretion of proinflammatory factors and cytokines, reducing oxidative stress, and inhibiting the brain cholinesterase, improves vascular endothelial task, regulates cerebral blood flow, and overall improves vascular dementia (Figure 2) (Table 1) (51). Other studies also pointed to these mechanisms (33, 54). Despite this evidence, this drug has not yet been approved for the treatment of vascular dementia (55).
2.1.4. Traumatic Brain Injury Dementia
Traumatic brain injury (TBI) is one of the leading factors of mortality and morbidity in many countries, especially developing countries (56, 57). A strike or pressure can cause this injury to the skull following a fall and accident or direct trauma to the head during sports and fights such as boxing and football. This trauma can cause various symptoms, such as Parkinson's-like symptoms, including tremors, gait and balance disorders, behavioral and psychiatric diseases, and sleep disorders. Concerning the specific anatomy of the brain and skull, it seems that this damage can lead to diffuse axonal injury, progressive neurodegeneration, and atrophy in different areas of the brain, especially vulnerable areas such as the hippocampus, amygdala, and frontal cortex, which have essential roles in cognitive processes and executive functions; thus, such damages can cause cognitive problems and dementia even years after the injury (56-59). As a result, research has sought to develop new treatments to ameliorate the symptoms and complications of this injury, but the FDA has yet approved no treatment; therefore, the pharmaceuticals are administered off-label (58-60). Studies showed that the pathology, brain changes, and cognitive impairment patterns following TBI are similar in aspects to Alzheimer's dementia. For example, TBI, like Alzheimer's, can affect short-term and long-term memory or cause behavioral and mood disorders. Aggregation of beta-amyloid plaques and neurofibrillary tangles, followed by neurodegeneration and atrophy in areas such as the medial temporal lobe and hippocampus, parahippocampal gyrus, thalamus, hypothalamus, and amygdala, are seen in both AD and TBI. However, this pattern of pathology differs from AD in some aspects. In addition, changes and destruction of cholinergic nerve pathways have been observed in TBI, similar to AD. Therefore, due to the similarities in the physiopathology and symptoms of TBI and AD, one of the drugs considered in the studies and prescribed off-label is AChEIs, especially donepezil (56-61). Yu et al. used transgenic mice to examine the effect and mechanism of donepezil for improving post-TBI memory impairment and found that it was effective in improving learning and spatial memory after injury and neurogenesis. However, it appeared that the drug's effectiveness was greater in memory impairment through mechanisms other than neurogenesis after injury (61). Furthermore, a meta-analysis by Wheaton et al. analyzed five studies that examined the effectiveness of donepezil alone and one study that examined the effectiveness of donepezil in combination with physostigmine and lecithin for improving cognitive symptoms after the acute stage of brain injury (four weeks after injury) at different disease severities, concluding that donepezil could improve attention and memory at different TBI severities (35).
Moreover, in a meta-analysis by Bengtsson and Godbolt which analyzed clinical trials examining the effect of AChEIs on TBI-induced cognitive impairment beginning at least 12 months after injury, it was found that donepezil at a dose of 10 mg had a significant effect on improving visual memory and learning. However, this effect was not observed at a dose of 5 mg (56). Campbell et al. made a retrospective comparison of the effect of donepezil (starting 5 mg daily and increasing to 10 mg daily seven to 10 days after starting treatment) with rehabilitation therapy on cognitive factors during the acute recovery phase of moderate to severe TBI among 129 patients (55 in the donepezil group and 74 in the rehabilitation group). They found that the two groups had no significant differences (Table 1) (36). However, many studies recommend a dose of 5 - 10 mg to treat TBI-induced cognitive impairment (58-60).
2.1.5. Multiple Sclerosis Cognitive Dysfunction
Multiple sclerosis is a demyelinating disease that can cause disability, especially in young people (62). One of the relatively common problems of this disease is cognitive impairment, which affects various areas of cognition such as working memory, executive function, episodic memory, and attention, and reduces the quality of life and even results in depression in the patients. Therefore, various studies have investigated the pathophysiology of this cognitive disorder and its treatment (62, 63). Evidence suggests that a defect in the hippocampal cholinergic system is one of the underlying mechanisms, similar to AD pathophysiology (64). Hence, donepezil is one of the drugs considered in studies (62, 63, 65). According to two studies by Shahpouri et al., donepezil could improve cognition, executive function, depression, and quality of life (62, 65). However, according to a study by O'Carroll et al., this drug did not significantly improve cognition (63). Therefore, more studies are needed to make a definite decision.
2.1.6. Schizophrenia Cognitive Impairment
Schizophrenia is a psychiatric disease with diverse symptoms such as hallucination and delusion. Impairments in different domains of cognition such as working memory, attention, executive function, and language skills are other important and influential symptoms in the daily life of people with the disorder. Therefore, various studies have tried to find a treatment for this cognitive impairment (66, 67). According to some radiological, autopsy, molecular, and histological evidence showing impairment in the cholinergic system and the nicotinic and muscarinic receptors in many cognitive areas such as the hippocampus formation, cortex, and basal ganglia, the effectiveness of acetylcholinesterase inhibitors, especially donepezil, has been considered in studies (67, 68). However, studies have shown mixed results. For example, Zhu et al. examined the effectiveness of donepezil 5 mg for 12 weeks in patients with chronic schizophrenia treated with antipsychotic drugs. The results showed that this drug could significantly improve cognition domains such as working memory and visual learning (66). However, another study by Hsu et al. found that donepezil could not significantly improve cognitive impairments (69). Therefore, further research is needed for the exact conclusion.
2.2. Donepezil and Non-dementia Diseases
2.2.1. Parkinson's Disease
According to studies, in addition to the applications of donepezil for different types of dementia, it can also play a role in some other non-dementia neurological diseases. Among them is the use of donepezil to improve balance and gait disorders caused by Parkinson's disease (12, 70).
Parkinson's is a neurodegenerative disease characterized by various symptoms, including tremors, imbalance, and gait disturbance. Consequently, these symptoms may reduce a person's quality of life, induce frequent falls and fear of falling, and even endanger their lives. Therefore, various studies were conducted to find the mechanisms of this disease and its treatment. According to studies, dopaminergic drugs, such as levodopa, which play a pivotal role in treating Parkinson's disease, do not appear to be effective in improving balance and gait disorders. Therefore, studies have proposed various theories for the physiopathology of this disorder, including the role of cholinergic neurons and branches in the structures and centers involved in the movement, such as the basal forebrain cholinergic complex (BFCC), pedunculopontine (PPN), and laterodorsal tegmental nuclei, cholinergic pathways in the brainstem and basal ganglia, and cholinergic projections to the thalamus. The role of cognitive processes such as attention, memory, and executive function in gait and keeping balance is also mentioned. Therefore, as mentioned in the section "Parkinson's dementia," the cholinergic system plays a fundamental role in cognitive processes (Figure 2) (12, 37, 70-74).
However, studies have shown diverse outcomes. A cross-over clinical study conducted by Chung et al. in 2010 examined the effectiveness of donepezil in preventing falls in 23 individuals with Parkinson's disease with a history of falls or near falls more than two times per week over 15 weeks and found that the number of falls per day in the donepezil group was approximately half that of the placebo group (37). Moreover, Stuart et al. conducted a cross-over clinical trial to evaluate the efficacy of a combination of cholinergic drugs (donepezil 5 mg) and dopaminergic drugs (levodopa) in the treatment of gait problems among 19 patients with Parkinson's disease over 50 years of age and treated with levodopa, with a score of 2 to 4 on the Hoehn and Yahr scale. They found that levodopa reduced the activity of the prefrontal cortex, which plays a vital role in executive function. Nevertheless, it did not happen in the combination of donepezil and levodopa. Also, the accuracy of performing two tasks simultaneously while using levodopa and donepezil increased compared to using levodopa alone. During the simultaneous use of donepezil and levodopa, gait function and its components, such as gait speed and stride length, were also improved compared to levodopa and placebo during single and binary activity (70). However, the meta-analysis by Chen et al. found that AChEIs did not substantially enhance gait and balance, despite that these medications appeared to be relatively effective in regulating steps during single activities (12). Moreover, the study by Mancini et al. (74) on 45 patients with Parkinson's disease, with relatively similar conditions to the study by Stuart et al. (70), showed that donepezil had no significant effect on improving motor and balance components in Parkinson's patients (Table 1) (74). In general, the findings tend to vary depending on the methods and measurement scales. These studies had a small sample size as well. As a result, larger sample sizes and more precise and sensitive scales are required for more accurate results.
2.2.2. Sleep Disorders
Donepezil may have a role in improving sleep disorders in healthy and Alzheimer's patients, Lewy dementia, autism spectrum disorder, obstructive sleep apnea, and mood disorders such as depression (13, 75-78). According to various studies, due to its impact on increasing the acetylcholine concentration in the brain and the critical role of this neurotransmitter in sleep processes, donepezil can increase the percentage of rapid eye movement (REM) sleep and regulate REM latency in individuals (13, 75, 79).
2.2.3. Mood Disorders
Donepezil and AChEIs have shown mood-modifying properties in animal and clinical trials. However, some research has refuted this efficacy, and the findings of specific studies have shown that these medicines cause depression, mania, or even exacerbation of the underlying condition (13, 80). In an animal study by Papp et al., using 96 rats in both stress and control groups, the anxiolytic, antidepressant, and cognitive-improving effects of donepezil (0.3 mg/kg intraperitoneally), rivastigmine, imipramine, and memantine were investigated. The results indicated the efficacy of donepezil in improving the symptoms of anxiety, depression, and cognition impairment (81). Moreover, in a case report by Lim et al., the antidepressant effect of donepezil at a dose of 5 mg as adjunctive therapy was noticed in a 62-year-old woman with a history of depression without any cognitive problems whose depression was resistant to routine depression treatments and even next-line drugs (82). However, as previously stated, some studies have shown inconsistent evidence regarding donepezil's efficacy in treating depression (80, 83). Reynolds et al., for example, in a clinical trial study, found that donepezil was ineffective in treating depression in 130 older adults with depression and mild cognitive impairment (38). There is an important theory about the antidepressant effects of donepezil: this drug follows the Janus-faced (u-shaped) dose-response curve. In other words, this drug shows antidepressant effects at low and usual doses and depression-like effects at higher doses (13, 39, 80). Various studies confirm this theory. For example, an animal study by Fitzgerald et al. with 160 mice showed that low doses of donepezil (0.02 to 0.2 mg/kg) had antidepressant properties (approximately equivalent to the usual dose of 5 - 10 mg/day for humans) and higher doses (2 mg/kg) had depression-like effects (Table 1) (39, 80).
The action mechanism of AChEIs, especially donepezil, in mood disorders and depression is unclear and seems complicated. However, according to studies, various mechanisms, such as the antidepressant effect of donepezil via the sigma-1 receptor, the anxiolytic, anhedonic, and antidepressant effect of this drug through nicotinic acetylcholine receptors, and regulation and interaction with other neurotransmitters like dopamine and serotonin have been suggested as possible mechanisms (Figure 2) (13, 81, 84).
Another application of donepezil in mood disorders includes improving cognitive impairment caused by selective serotonin reuptake inhibitors (SSRIs). A clinical trial study by Hosseini et al. examining the efficacy of donepezil in improving cognitive impairment induced by citalopram, sertraline, and fluoxetine in depressed patients aged 20 to 50 years confirms this effect (Table 1) (40). Altogether, it seems that donepezil can affect mood disorders differently, but more studies are needed for more accurate and comprehensive conclusions.
2.2.4. Autism Spectrum Disorder
Autism spectrum disorder (ASD) is a neurodevelopmental disease that has various degrees characterized by symptoms such as poor communication and social interaction, limited and repetitive interests and behaviors, language skill problems, and cognitive impairments (42, 85, 86). Even though medications like risperidone, atomoxetine, aripiprazole, and SSRIs have been approved and are already in use, they are not considered successful in alleviating the core symptoms of the disease. On the other hand, this disease's risk factors and pathophysiology are not known precisely, but it seems that various genetic and environmental factors are influential (42, 85, 87). Therefore, studies on the pathophysiology and drugs affecting this disease are still ongoing. Hence, donepezil is one of the candidate drugs that can be effective in improving the symptoms of this disease by some mechanisms (41, 42, 75).
A study by Karvat and Kimchi examined the effect of donepezil systematically (intraperitoneally) and directly injected into the dorsomedial striatum (DMS) (which is equivalent to the caudate nucleus in humans) on autism-related behaviors in BTBR T + tf/J mice (whose symptoms were very similar to autistic patients). They found that systemic administration of donepezil could improve performance, social interaction, and cognitive flexibility dose-dependently. It was also found that direct administration of DMS improved cognitive flexibility and social factors in autistic mice (85). In another animal study by Kim et al., which examined the effect of subchronic use of donepezil on behaviors and symptoms of valproic acid-induced autistic mice, donepezil could significantly improve the symptoms and behaviors of autistic mice, such as abnormal social behaviors and repetitive behaviors, hyperactivity, and cognitive inflexibility (87). A clinical trial study by Gabis et al., which examined the effect of a combination of donepezil and choline for 12 weeks compared with a placebo on language skills improvement among 60 people with autism aged 5 to 16 years, found that the combination has a significant effect on autistic children under 10 years old compared to the placebo. While the drug was safe regarding side effects, it significantly improved language skills (41). A systematic review by Rossignol and Frye which analyzed the effect of anti-Alzheimer's drugs on the treatment of autism core symptoms, showed that donepezil had an improvement role in some studies (five out of seven studies). Nevertheless, due to contradictory results and differences in the evidence level of studies, no definite conclusion was reached (Table 1) (42).
Defects in the cholinergic system play a fundamental role in the pathophysiology of autism. Histological studies of dead autistic people, for example, have shown a reduction in nicotinic and muscarinic receptors in the parietal, frontal, and temporal cortices, thalamus, and hippocampus. Radiological studies have shown similar results. Therefore, it seems that donepezil can improve the symptoms of autism by regulating acetylcholinesterase and affecting acetylcholine and cholinergic receptors (41, 42, 85, 87).
According to studies, another proposed treatment for ASD is a change in the state of the brain to hypomania because the pathophysiology and symptoms of the two diseases are opposite in many aspects. For example, ASD has low social engagement, low cerebral vasopressin, high cortisol, and low mirror neuron activity, but mania, particularly hypomania, has the reverse characteristic (88). On the other hand, donepezil has been shown in certain studies to produce hypomania by altering the ratio of acetylcholine to dopamine and binding to nicotinic receptors. Therefore, it seems that this effect as one of the possible mechanisms of donepezil for improving ASD needs further investigation (88-91).
2.2.5. Immune System and Infectious Disease
According to studies, in addition to therapeutic applications in neurological and psychiatric diseases, donepezil can have different effects on the immune system through different mechanisms. Donepezil can affect innate and acquired immune systems through different pathways (14, 92, 93).
Studies show that the cholinergic system plays a role in regulating the immune system and the inflammatory processes. For example, the cholinergic anti-inflammatory pathway (CAP), the efferent branch of the brain-immune loop, initiates anti-inflammatory responses and modulates inflammation from various pathways by secreting acetylcholine. Acetylcholine reduces the production of inflammatory cytokines via modulating nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) gene expression in macrophage cells, which play a fundamental role in the immune system's response to pathogens and inflammatory processes by binding to the alpha-7 nicotinic receptors on these cells. Acetylcholine also modulates the immune system and reduces neutrophil leakage by binding to muscarinic receptors in many cell populations (Figure 1) (14, 92, 94-96).
Besides, studies indicated the anti-inflammatory and neuroprotective effects of donepezil. Evidence shows that 5 - 20 μM donepezil influences microglial cells and suppresses their inflammatory activation. In addition, the CAP has a major role in these effects. Furthermore, the decline of the microglial production of NO and TNF-α and suppression of iNOS, IL-1β, and TNF-α gene expression by donepezil were reported. It seems that suppression of microglial activation by donepezil is independent of acetylcholine and its receptor (Figure 1) (97).
On the other hand, in many neurodegenerative, autoimmune, and inflammatory diseases, the balance of the innate immune system and its components, such as cytokines, is disturbed. Sochocka et al., in an in vitro study, investigated the effect of different doses of donepezil on different innate immune mechanisms, such as resistance of peripheral blood leukocytes (PBLs) to viruses (by examining the replication of vesicular stomatitis virus (VSV) and herpesvirus type 1 (HSV-1) in cell lines taken from healthy and AD patients at different ages) and the production of cytokines (by direct measurement of cytokines such as IFNs and TNF-α, as well as the activation of NF-KB signaling, which regulates cytokine secretion, by immunocytochemical staining). They found that donepezil had a regulatory role in the innate immune system and could affect the antiviral resistance of PBLs, especially in the leukocytes of healthy individuals, and the production and secretion of cytokines in the leukocytes of both healthy individuals and Alzheimer's patients (98, 99). In addition, a similar study studied the effect of two drugs, Ginkgo biloba special extract (EGb 761) and donepezil, both used for AD treatment, on innate immunity mechanisms, including natural antiviral resistance of human leukocytes in vitro and NF-κB activation. Thirty healthy blood samples were collected from 15 individuals with resistant leukocytes to VSV and 15 individuals with sensitive leukocytes. It was found that 10 - 50 μg/mL donepezil and 25 - 100 μg/mL EGb761 increased the resistance of human leukocytes and reduced the activation of transcriptional factor NF-κB. Furthermore, a relationship was seen between NFκB activation and the level of leukocyte resistance (Table 1) (Figure 1) (44).
According to studies, the cholinergic system seems to play an influential role in modulating lung inflammation, especially in infections, and maintaining normal lung function. Increased secretion of acetylcholine following lung inflammation can help modulate inflammation and repair damaged lung tissue by various pathways, such as shifting the macrophages population to M2 macrophages, which secrete anti-inflammatory cytokines and play an anti-inflammatory role in inflammatory processes, regulating the differentiation and growth of airway cells to improve the function of the epithelium of these ducts, which are altered by inflammatory cytokines, reducing neutrophil leakage from these ducts, and increasing cholinergic lymphocytes in the lungs, which play an influential role in regulating inflammatory processes (Figure 1) (14). A retrospective study by Abe et al. also showed this effect. This research, which analyzed the data from 25,602 elderlies hospitalized in Japan for almost two years with a diagnosis of pneumonia, revealed that donepezil decreased death by 64%. People receiving donepezil for dementia (about 578 individuals) showed considerably lower death rates than those with dementia who did not take donepezil and those without dementia. Therefore, this drug, which increases acetylcholine levels, was considered an independent factor in reducing pneumonia mortality among the elderly in this study, consistent with previous studies (Table 1) (43).
Moreover, patients treated with donepezil showed declined death risk from pneumonia and myocardial infarct, increased vascular function, and suppressed stroke risk. The studies showed that fatal acute respiratory distress syndrome (ARDS), sepsis, cytokine storm, cardiovascular damage, disseminated intravascular coagulation (DIC), and acute kidney damage could be inhibited via this drug. These effects seem to be due to the immunomodulatory effects of donepezil on innate and acquired immune systems (14).
Similarly, a decline in the risk of death was reported in patients treated with donepezil after COVID-19 infection. In addition to the mechanisms mentioned above, this effect appears to be due to the effects of donepezil on the sigma-1 receptor (14). Coronaviruses replicate in an altered membrane portion of the endoplasmic reticulum (ER), contribute to ER stress in the host cell, and activate pathways to accommodate the host cells for viral demands. The regulation of ER remodeling and ER stress response may play a significant role in virus-host interaction and infection treatment. Consequently, the sigma-1 receptor (Sig-1R), a membrane chaperon with an upstream modulatory function in ER stress, has been studied as a potential treatment for COVID-19 patients. Sig-1R modulates vital mechanisms of the adaptive host cell stress response and is involved in the early steps of viral replication. Using Sig-1R as a target in therapeutic approaches seems not to decrease viral replication. However, it might be a cross-reaction with early steps of virus-induced host cell reprogramming, helping decline the course of infection, inhibiting the severity of the disease, or even making a chance to develop a preventive immune response. Therefore, it seems that drugs, such as fluvoxamine, donepezil, and hydroxychloroquine, which have relatively good binding to this receptor, can be effective in modulating and treating the disease through mechanisms such as inhibiting the interaction of virus proteins with this receptor and subsequently inhibiting intracellular signaling (Figure 1) (100, 101).
There are also similarities between the pathophysiology of AD and COVID-19. In both diseases, inflammatory processes, such as increased proinflammatory cytokines like IL-1 and IL-6 and increased cytoskeleton-associated protein 4 (CKAP4) and galectin-9 (GAL-9), are seen (102). Therefore, it seems that donepezil, due to its immunomodulatory effects on the pathophysiology of AD, as described in detail in the section "AD and donepezil," can be effective in treating COVID-19. However, more preclinical and clinical studies are needed (102).
2.3. Current Challenges and Future Prospects of Donepezil
Donepezil is an important cholinesterase inhibitor for treating AD, but its therapeutic efficacy varies from 20 - 60%, and patients with AD respond differently to it. Various studies showed that genetic factors affect pharmacokinetics and pharmacodynamics differently, leading to different clinical efficacies. Several studies indicated the association between the therapeutic efficacy of donepezil and hepatic enzymes gene polymorphisms like CYP2D6 and CYP3A4 (7). Apolipoprotein E (APOE) alleles like APOE E3 also affect the efficacy of donepezil. Therefore, these personal differences must be considered for achieving better clinical outcomes (7).
Another challenge in treating diseases like AD is their different symptoms and pathways. Therefore, it is necessary to use diverse drug combinations to control the disease, which could reduce the capacity of patients and increase side effects and drug interactions. Various studies have been conducted to design new derivatives of donepezil to affect different receptors and pathways. The benzylpiperidine and dimethoxy indanone are components of the donepezil structure that could bind to or replace with compounds such as pyridyl methyl piperidine, benzylpiperazine, pyrimidyl piperazine, heterocyclic rings, ferulic acid, flavonoids, and curcumin, making derivatives with new properties such as anti-oxidative, anti-dementia, and monoamine oxidase inhibition-like effects (4). Therefore, more studies are needed to obtain better therapeutic responses and fewer side effects in the future.
3. Conclusions
Although many studies have emphasized the efficacy of donepezil in treating AD, various clinical and preclinical studies showed that it could positively affect other dementias, such as Parkinson's disease, vascular dementia, and other neurological, psychiatric, and even infectious diseases. These effects of donepezil seem to be exerted through other mechanisms like sigma-1 receptor and cholinergic receptors signaling activation and inflammation processes modulation, in addition to inhibiting AChE and increasing acetylcholine.
In general, more comprehensive and accurate studies are needed to make better decisions about the efficacy of this drug in other diseases. It is also recommended to consider personal differences in clinical responses and apply new derivations with multi-efficacy in further studies.