1. Background
Prostate cancer is the most common cancer among men and the fifth most frequent cause of cancer-related death globally (1). The treatment of prostate cancer is associated with the cancer stage and can include surgical resection, radiotherapy, chemotherapy, and anti-hormone therapy.
Androgen deprivation therapy (ADT) is a standard first-line option for patients with advanced castration-sensitive prostate cancers. Bilateral orchiectomy (surgical castration) or medical castration can be considered for performing ADT in prostate cancer patients. Medical castration is associated with decreases in gonadal testosterone production via inhibiting the hypothalamic-pituitary axis. The continuous administration of a gonadotropin-releasing hormone (GnRH) agonist is the most commonly used modality, which restrains the production of luteinizing hormone and testicular androgens.
Androgen deprivation therapy is accompanied by numerous side effects which dramatically diminish patients’ quality of life. The most frequently reported side effects to include loss of lean body mass, sexual dysfunction, loss of libido, vasomotor instability manifested by hot flashes, behavioral and neurologic effects, fatigue or lack of energy, etc. On the other hand, long-term administration of ADT leads to cardiovascular and metabolic abnormalities and loss of bone mineral density, which results in osteoporosis or bone fracture (2). Various agents such as gabapentin or venlafaxine (2) have been addressed for treating ADT-induced vasomotor symptoms in men; however, benefits must be balanced against treatment-related side effects.
In 2010, Irani et al. (3) conducted a study on 311 men who had been treated with a GnRH agonist for six months while complaining of hot flashes, a minimum of 14 hot flashes per week. In this study, enrolled men were randomly assigned to receive either medroxyprogesterone (20 mg/day), cyproterone (100 mg/day), or venlafaxine (75 mg/day). After 12 weeks’ follow-up, all three regimens showed a significant decrease in the frequency of hot flashes compared with baseline.
In addition, psychiatric complications such as mood and sleep changes and, consequently, quality of life impairment are still a concern in prostate cancer patients receiving ADT.
Melatonin (N-acetyl-5-methoxytryptamine) is a pineal gland-secreted hormone, and its synthesis is associated with circadian rhythm regulation. Multiple biological functions have been reported for melatonin in the body, for instance, the role in sleep induction and regulation, homeostasis, vasoregulation, and even immunomodulation (4). Melatonin was suggested to have sedative, anxiolytic, analgesic, anti-hypertensive, antioxidant, non-inflammatory, and oncostatic properties. It also has possible anti-depressive effects and cognition improvement due to its impacts on central circadian regulation (5).
Garzon et al. (6) evaluated the effect of melatonin administration (5 mg daily for two months) on sleep and behavioral disorders in the elderly community-living population, and they found that melatonin administration significantly improved sleep and behavioral problems in the elderly population and facilitated discontinuation of therapy with conventional hypnotic drugs.
In the melody trial (7), by considering the mechanism of cancer-induced depression and clock-gene phenotypes in breast cancer patients, melatonin was suggested as a potential agent for treating depression, anxiety, cognitive dysfunction, and sleep disorder-induced by cancers. However, they proposed that larger and well-designed clinical trials are needed for any conclusion.
2. Objectives
Based on the promising results extracted from melatonin administration in different studies conducted on patients suffering from psychological problems such as depression and mood changes, we aimed to assess the effect of melatonin on sleep disorders and mood changes in prostate cancer patients who were receiving ADT while complaining of mood and sleep problems.
3. Methods
The study methodology was a randomized, double-blind placebo-controlled clinical trial conducted in the hematology-oncology center of Omid hospital, Isfahan, Iran, during 1-year patient recruiting time (Oct 2019 - Oct 2020). Omid hospital is one of the tertiary and university-affiliated cancer centers located in the center of Iran, and it is well-equipped for treating cancer and its complications.
3.1. Participants
We include adult men (older than 18 years) diagnosed with prostate cancer who only received ADT as a part of their treatment modalities. Before starting patient recruitment, all patients considered for recruitment were screened by the hospital anxiety and depression scale (HADS), translated and validated in Persian by Montazeri et al. (8).
This scale is a fourteen-item used for measuring psychological morbidity in patients with cancer ranging from zero to three for each item. For not involving psychological patients who needed psychiatrists’ consultation, we only included patients who had cumulative total scores of more than 11 in the early assessment while complaining of mood or sleep changes during the last month. We just included patients who were able to take oral melatonin.
Exclusion criteria were as follows: Patients with metastatic prostate cancer, patients with a tumors history other than prostate cancer, and those patients who were performing concurrent chemotherapy, radiotherapy, or surgery with their ADT. Patients who were taking specific medications such as propranolol due to its central nervous system (CNS) effects, warfarin due to its high risk of drug interaction and adverse effects, and several CNS pharmacological drug class such as selective serotonin reuptake inhibitors (SSRIs), norepinephrine reuptake inhibitors (SNRIs), antipsychotic, anti-convulsant, and monoamine oxidase inhibitors (MAOIs) inhibitors. We also excluded patients who were receiving hypnotic drugs like benzodiazepines or non-benzodiazepines.
3.2. Ethical Considerations
The Medical Research Ethics Committee of Isfahan Medical University has approved this clinical research (approval code: IR.MUI.RESEARCH.REC.1399.143). We also registered the study protocol on the Iranian registry website for a clinical trial (ID: IRCT20180722040556N5). All participants read the informed consent form, and any ambiguities were thoroughly explained. Participants who chose to proceed with the study signed the consent form.
3.3. Concealing and Randomization
This double-blinded study was randomized by the block randomization method. The block randomization software (9) allocated the codes for each group in a sealed opaque envelope according to our statistical information, such as sample size, number of groups (2 groups, intervention group, and placebo group), and size of the block. Razak® Pharmaceutical Company provided the melatonin 3 mg tablets and an identical placebo. The placebo tablets contained the same ingredients and excipients (including microcrystalline cellulose, calcium phosphate dibasic dihydrate, and magnesium stearate except the effective agent (melatonin), and they were produced in the same shape, size, color, and package as melatonin tablets. Each pack of tablets contained 60 tablets and was labeled using the generated code series by the study’s principal investigator. None of the patients, the investigators, responsible physicians, and the statistical analyzer were aware of the allocated patients’ codes.
All participants who had consensually decided to participate in our clinical trial were observed for one week. During this time, we collected baseline data, screened by HADS, and attempted to assess participants' compliance and familiarize them with the questionnaires. Then patients were considered for baseline examination by all questionnaires, including the Pittsburgh sleep quality index (PSQI) (10), Beck Depression Inventory (BDI) (11), and the Hamilton Anxiety Rating Scale (HAM-A) (12). After that, in the second week of follow-up, patients were considered to receive either melatonin (3 mg twice daily) or an identical placebo randomly for four weeks. At the end of the 4-week follow-up, again, patients filled the all aforementioned questionnaires to consider for comparison with baseline data.
3.4. Intervention
In the present study, 43 patients (21 and 22 patients in melatonin and placebo, respectively) were recruited, and their baseline characteristics and demographic data, including age, history of medical disease, history of medications, history of the type of therapy, and type of ADT were collected.
3.5. The Assessed Questionnaires
For assessing the sleep quality during the 1-month intervention, the self-report PSQI questionnaire was applied. The questionnaire consists of 19 individual items in 7 different domains consisting of subjective sleep quality, habitual sleep efficiency, sleep disturbances, sleep aid medication usage, and daytime dysfunction. Each PSQI questionnaire’s questions were rated from 0 to 3 (0: Optimum score, 3: The worst score) and took 5 - 10 minutes to complete (13).
The second 21-question multiple-choice self-report questionnaire was BDI developed to assess the severity of depression originating from the patient's thoughts, not a psychodynamic perspective. The standardized cutoffs of depression severity are as follows: The total number classified depression severity to 0 - 13 (minimal), 14 - 19 (mild), 20 - 28 (moderate), and 29 - 63 (severe) (14) and higher cumulative numbers mean severe depressive symptoms.
Finally, for rating the severity of a patient’s anxiety, the 14 items HAM-A questionnaire was applied (15). In this questionnaire, the cumulative total scores of questions indicate the severity of anxiety among patients. A total score of 17 or less indicates mild severity, while scores between 18 and 24 indicate mild to moderate severity, and scores between 25 and 30 indicate moderate to severe severity.
3.6. Translation and Validation of Questionnaires
The English version of the questionnaires had been previously logically validated and used in previous studies (16-18). As two questionnaires, BDI and PSQI have been translated and validated in previous studies by Stefan-Dabson et al. (19) by Cronbach's alpha of 0.90 and Ahmadi et al. (20) by Cronbach's alpha 0.89; respectively, we applied the Persian version of these questionnaires for our assessment. However, the reliable version of the HAM-A questionnaire was not accessible, and we decided to consider the original format for translation and validation before applying it to our study. For Persian translation and validation of the HAM-A questionnaire, two independent bilingual translators first translated the English questionnaire into Persian. After that, to perform a backward translation, two independent translators were assigned to translate the questionnaire into Persian again, while they did not become aware of the intended concepts of the questionnaire measures.
Furthermore, we gathered an expert committee that included experts familiar with the methodology and concept of the study to develop the original questionnaires. The expert committee reviewed the whole versions of translations and determined whether the translated and original versions meet semantic, experiential, idiomatic, and conceptual equality. After resolving any discrepancies, members of the expert committee reached a consensus to develop a pre-final translated questionnaire version.
To determine the face and content validity, a panel of experts, including two pharmacists, two general physicians, one Persian literature specialist, ten oncologists, and two psychologists, were selected to decide which items were presented in suitable words while maintaining the whole theoretical construct. Thereafter, the new questionnaire items were considered to pass through a preliminary pilot test-retest method in 20 selected patients during a 2-week interval. Then the subsequent revisions were analyzed for internal consistency assessment using the coefficient alpha, also known as Cronbach's alpha (21).
3.7. Evaluation of Side Effects
The Common Terminology Criteria for Adverse Events (CTCAE) standard version 5 was used to assess side effects (22). In this study, we focused on the side effects of melatonin or placebo on the central nervous system (CNS) and gastrointestinal system. Also, for evaluating daytime sleepiness, Epworth Sleepiness Scale scores were used (23), which is a self-administered questionnaire with eight questions ranging from 0 - 24.
3.8. Data Analysis
Consistent with the study by Chen et al. (24), in which the treatment ratio of the intervention and control groups was 7.2 ± 3.3 and 5.5 ± 4, respectively, the sample size was estimated as 29 patients in each group. The minimum sample size was increased to 33 participants, considering a power of 80%, a confidence interval of 0.95, a significance level of 0.05, and a dropout rate of 10%.
(N = 33)
After follow-ups, a per-protocol analysis was conducted to compare the data of the remaining patients. The normality of patients' demographic and clinical characteristics was checked by the Kolmogorov-Smirnov test. The differences between the two groups' parameters were assessed by statistical tests including chi-squared, independent t-test, and Fisher's exact test. We used a Paired-sample t-test to analyze the mean scores of BDI, HAM-A, and PSQI questionnaires during pre and post-intervention. Also, we found the internal consistency of the HAM-A questionnaire by Cronbach's alpha formula. The Statistical Package performed all analyses for Social Sciences (SPSS) version 20 software, and P-values less than 0.05 were considered significant differences.
4. Results
4.1. Internal Consistency of HAM-A Questionnaire
After calculating Cronbach's alpha as a test score reliability coefficient for the HAM-A questionnaire, it was estimated to be 0.84 (between 0.6 and 1), indicating the sufficient reliability and efficiency of the Persian-translated version of the HAM-A questionnaire.
4.2. Clinical and Demographic Characteristics
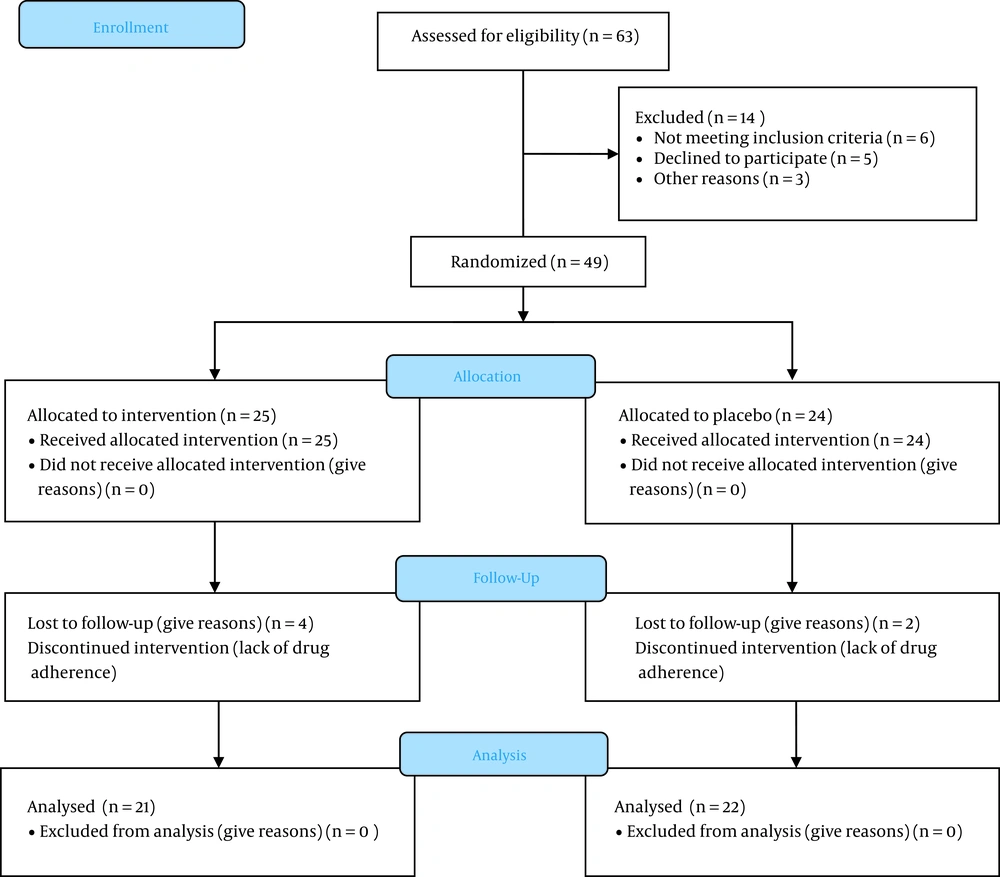
In this study, during the first attempts, 63 patients were initially evaluated for fulfilling inclusion criteria, as shown in Figure 1; however, in the end, 20 patients were excluded due to not being willing to participate, disease progression, or death.
After that, 43 remained patients were randomly divided into melatonin (21 patients) or placebo groups (22 patients). The clinical and demographic characteristics of patients are shown in Table 1. There were no significant differences in baseline characteristics in terms of age, baseline prostate-specific antigen (PSA), underlying diseases, family history of cancer, baseline mean HADS scores, and previous treatment protocols between the two compared groups. The mean age was 70.57 ± 6.77 years in the melatonin group and 68 ± 17.44 years in the placebo group. The two groups had no significant differences in the above variables (P > 0.05).
| Variables | Melatonin Group (N = 21) | Placebo Group (N = 22) | P-Value |
|---|---|---|---|
| Age | 70.57 ± 6.77 | 68 ± 17.44 | 0.53 |
| Baseline PSA | 30.55 ± 15.24 | 29.54 ± 14.25 | 0.45 |
| Baseline HADS Score | 10.2 ± 2.2 | 10.7 ± 1.9 | 0.45 |
| Family history of cancer | 6 (31.6) | 5 (22.7) | 0.55 |
| Comorbidities | |||
| Type 2 diabetes | 4 (21.1) | 3 (13.6) | 0.53 |
| Dyslipidemia | 4 (21.1) | 6 (27.3) | 0.64 |
| Hypertension | 4 (21.1) | 2 (9.1) | 0.28 |
| Ischemic heart disease | 2 (10.5) | 1 (4.5) | 0.46 |
| Others b | 5 (31.6) | 9 (40.9) | 0.33 |
| Treatment history | 0.24 | ||
| ADT | 7 (36.8) | 6 (27.3) | |
| ADT + radiotherapy | 7 (36.8) | 8 (36.4) | |
| ADT + surgery | 4 (21.1) | 2 (9.1) | |
| ADT + radiotherapy + surgery | 1 (5.3) | 6 (37.3) | |
| Medications used for ADT | 0.58 | ||
| Goserelin | 9 (47.4) | 9 (40.9) | |
| Triptorelin | 4 (21.1) | 10 (45.5) | |
| Triptorelin + abiraterone acetate | 2 (10.5) | 1 (4.5) | |
| Goserelin + abiraterone acetate | 1 (5.3) | 0 (0) | |
| Triptorelin + bicalutamide | 1 (5.3) | 0 (0) | |
| Goserelin + bicalutamide | 1 (5.3) | 1 (4.5) | |
| Triptorelin + flutamide | 1 (5.3) | 1 (4.5) |
Baseline Clinical and Demographic Characteristics of Enrolled Patients (N = 43) a
4.3. Pittsburgh Sleep Quality Index
Before implementing any intervention, the mean PSQI scores in the melatonin and placebo groups were 13.95 ± 3.47 and 13.05 ± 4.89, respectively. Also, after 4-week follow-ups, the indices decreased to 10.67 ± 3.18 and 12.14 ± 4.4 for the melatonin and placebo groups, respectively. The results showed that in both melatonin and placebo groups, after 4-week follow-ups, there was a significant difference between mean PSQI scores from baseline (within-group analyses). Also, the independent t-test showed that the inter-group differences were statistically significant (P-value = 0.04). On the other hand, in both melatonin and placebo groups, the general score of sleep indices improved before and after the intervention; however, the beneficial effects were seen as more significant in the melatonin group.
As shown in Table 2, melatonin administration significantly improved mean PSQI scores in four domains of sleep quality, sleep Efficacy, sleep latency, and daytime.
Except for the use of sleep aid medication, melatonin administration significantly improved the PSQI scores in most domains of the questionnaire, showing superiority in comparison with the placebo group. In addition, data from patients receiving placebo tablets showed that placebo administration effectively decreased mean PSQI scores during 4-week follow-ups in term of sleep latency, disturbance, and duration domains. In addition, in the placebo group, the daytime dysfunction of patients significantly worsened after 4-week follow-ups, while it was significantly improved in the melatonin group.
| PSQI Domains/Time of Intervention | Melatonin Group (N = 21) | Placebo Group (N = 22) | P-Value Between-Group |
|---|---|---|---|
| Sleep Quality | |||
| Before | 1.14 ± 0.65 | 1.22 ± 0.81 | 0.25 |
| After | 0.52 ± 0.67 | 0.95 ± 0.48 | 0.03 |
| P-value within groups | 0.001 | 0.083 | |
| Sleep latency | |||
| Before | 0.8 ± 0.51 | 1.13 ± 0.63 | 0.46 |
| After | 0.19 ± 0.4 | 0.5 ± 0.51 | 0.001 |
| P-value within group | 0.001 | 0.001 | |
| Sleep duration | |||
| Before | 1.23 ± 0.76 | 1.31 ± 0.99 | 0.13 |
| After | 0.90 ± 0.53 | 1 ± 0.97 | 0.11 |
| P-value within group | 0.01 | 0.051 | |
| Sleep efficacy | |||
| Before | 0.57 ± 0.97 | 0.63 ± 0.90 | 0.66 |
| After | 0.23 ± 0.43 | 0.5 ± 0.8 | 0.002 |
| P-value within group | 0.049 | 0.37 | |
| Sleep disturbance | |||
| Before | 1.06 ± 0.28 | 0.99 ± 0.41 | 0.14 |
| After | 0.86 ± 0.28 | 0.86 ± 0.37 | 0.21 |
| P-value within group | 0.001 | 0.01 | |
| Hypnotic drugs b | |||
| Before | 0.14 ± 0.65 | 0.13 ± 0.63 | 0.94 |
| After | 0.14 ± 0.65 | 0.13 ± 0.63 | 0.94 |
| P-value within group | 1 | 1 | |
| Daytime dysfunction | |||
| Before | 0.19 ± 0.60 | 0.36 ± 0.78 | 0.17 |
| After | 0.09 ± 0.43 | 0.68 ± 0.94 | 0.001 |
| P-value within group | 0.32 | 0.05 |
The PSQI Domains Comparison at the Baseline and After 4-week Follow-ups Between Melatonin and Placebo Groups a
4.4. Hamilton Anxiety Rating Scale
As shown in Table 3, before starting the intervention, the Hamilton Anxiety Rating Scale (HAM-A) mean total scores in the melatonin group were 20.17 ± 8.7, and in the placebo group was 18.54 ± 5.2. At the end of the intervention, these mean total scores had been ameliorated in both groups (17.29 ± 6.5 in the melatonin group and 16.54 ± 5.3 in the placebo group).
| Questionnaire (Cumulative numbers)/Time of Intervention | Melatonin Group (N = 21) | Placebo Group (N = 22) | P-Value* (Inter-group) |
|---|---|---|---|
| PSQI | |||
| Before | 13.95 ± 3.47 | 13.05 ± 4.89 | 0.055 |
| After | 10.67 ± 3.18 | 12.14 ± 4.4 | 0.04 |
| P-value within group | 0.001 | 0.023 | |
| HAM-A | |||
| Before | 18.33 ± 7.36 | 17.23 ± 5.9 | 0.06 |
| After | 16.14 ± 6.5 | 15.64 ± 6.4 | 0.49 |
| P-value within group | 0.001 | 0.001 | |
| BDI | |||
| Before | 16.43 ± 7.78 | 12.45 ± 5.63 | 0.12 |
| After | 12.33 ± 5.88 | 11.05 ± 4.91 | 0.32 |
| P-value within group | 0.003 | 0.001 |
There was no significant difference between the two groups at the beginning and the end of the study comparison to each other. However, both groups experienced a significant reduction in HAM-A total score after implementing the intervention as melatonin or placebo (P < 0.05). There is a significant decrease in anxiety severity after intervention in the melatonin group.
4.5. Beck Depression Index
Before the intervention, BDI means the total score was 17.64 ± 7.9 in the melatonin group and 12 ± 5.9 in the placebo group. At the end of the intervention, these mean total scores have been ameliorated in both groups leading to a mean total amount of 13.41 ± 5.9 in the melatonin group and 10.77 ± 5 in the placebo group. Although total changes in cumulative scores in both intervention groups decreased after 4-week follow-ups, the difference between the two groups of melatonin and placebo was not statistically significant (see Table 3).
4.6. Adverse Effects
According to CTCAE version 5 (22), no noteworthy adverse reaction was reported by 6 mg daily administration of melatonin for four weeks. However, there were 3 cases of excessive daytime sleepiness, defined by scores between 7 - 15 based on the Epworth sleepiness scale, in the melatonin group, which was not statistically significant between the two comparative groups.
5. Discussion
As our knowledge serves, for the first time in our study, we demonstrated that melatonin supplementation during ADT in patients suffering from prostate cancer led to attenuation in sleep quality indices, including sleep latency. Furthermore, melatonin can be suggested as a safe and effective agent to be considered for prostate cancer patients suffering from ADT adverse effects especially sleep problems.
Androgen deprivation therapy is the main therapeutic approach for patients suffering from prostate cancer in different stages of the disease, including disseminated disease or the setting of adjuvant or neoadjuvant therapy. Regardless of the beneficiary, a wide range of side effects that negatively affect the quality of life and may necessitate a change in therapy has been reported by ADT. One of the most reported adverse events during ADT was noted as emotional, cognitive, and sleep changes due to omitting androgen levels (25). For example, treatment with ADT has also been noted to be associated with an increased risk of inducing depression and anxiety (26).
In a systematic review and meta-analysis that included results from 18 studies with 169000 individuals, there was a 41 percent increase in an analysis of 79000 men aged less than 65 years treated with ADT (three years’ cumulative incidence 4.1 versus 3.5 percent) (27).
Melatonin has been well documented as a physiologic agent that has the potency of ameliorating the circadian rhythm in order to relieve sleepiness complications. There has been an increased interest in melatonin administration in all aspects of sleep function/disorders in different disease conditions such as sleep-related breathing disorders, neurodegenerative disorder, autism spectrum disorders (28), depression (29), premenstrual dysphoric disorder (30), attention-deficit/ hyperactivity disorder (31).
Based on the previous promising effects of melatonin administration in the treatment of various medical conditions, we designed a study to examined the possibility of melatonin effects on the management of psychological and sleep problems associated with ADT.
As it was predictable, in our study, PSQI scores indicated sleep quality decreased significantly at the end of the intervention, and most of the PSQI domains have been improved after 4-week follow-ups. The use of exogenous melatonin as a nighttime sleep aid has been extensively researched in many studies (32), showing benefits in sleep onset, sleep maintenance, and total sleep time. The potential benefits may vary with the dose, timing of administration, and its relation to the individual’s circadian predisposition and also the age of the person. A 2020 systematic review identified 12 meta-analyses of placebo-controlled randomized trials (3 to 13 trials per the study, the largest analysis included 1315 patients) and concluded that melatonin resulted in a small improvement in sleep latency and total sleep time, with a lack of evidence on whether the effects will be clinically meaningful. However, our results were in favor of other research on non-cancer patients. Significant statistical differences have been indicated in total scores and sleep latency, efficacy, and daytime function domains.
In the randomized, double-blind, placebo-controlled study conducted on 95 postmenopausal women with prior history of stage 0 - 3 breast cancer, the possible efficacy of melatonin supplementation, 3 mg oral melatonin for 16 weeks, was assessed. Melatonin supplementation significantly improved subjective sleep quality domains, including sleep quality and daytime dysfunction measured by the PSQI questionnaire. The results of sleep quality enhancement in patients with prostate cancer while receiving ADT were in favor of menopausal breast cancer patients with a history of anti-hormone therapy (24) and were comparable with non-cancer patients' evaluation in different studies (33-35).
On the other hand, the role of melatonin in the pathophysiology of psychological diseases such as bipolar, major depression, or seasonal affective disorders is now a matter of interest. In a review article published in 2006 (36), melatonin has been introduced as a “state marker” and a “trait marker” for mood disorders. They indicated that the level and timing of melatonin secretion had been altered in numerous psychological disorders. Today, agomelatine (37), a selective melatonin receptors agonist and 5-HT 2B and 5-HT 2C receptors antagonist, has been proposed to have a comparative efficacy with venlafaxine (38) (75 - 150 mg) and is more effective than fluoxetine (20 - 40 mg) and sertraline (50 - 100 mg) (39) in the treatment of major depression. Furthermore, melatonin supplementation would be a reasonable option for treating ADT-induced mood disorders in prostate cancer patients.
According to the Beck depression questionnaire, the results of our study showed that melatonin supplementation was not able to attenuate the severity and degree of depression in prostate cancer patients receiving ADT during 4-week follow-ups. However, the results vividly demonstrated that melatonin was able to decrease the total scores of the Beck depression questionnaire, but due to limitations in the methodology of the study, including the short duration of follow-ups and low sample size, it failed to show statistical differences with the placebo group. In the case of evaluating the positive effects of melatonin supplementation on depression scores in future studies, this drug may find a unique place in treating depressive cancer patients due to its safety profile and favorable adverse effects.
In addition, we failed to show any significance between the degree and severity of anxiety evaluated by the HAM-A questionnaire.
However, there are several animal studies have addressed the protective effects of melatonin on alleviating anxiety-like behaviors in animal models (40, 41).
For example, Wang et al., in 2021 (41), revealed that melatonin administration attenuated sleep deprivation-induced anxiety-like behaviors in an animal model. They found that melatonin may play its role by ameliorating oxidative stress and the NF-κB pathway, reducing neuro-inflammation and excessive apoptosis.
The role of melatonin administration in the cancer-induced complications’ alleviation, or mostly ADT-induced anxiety and depression, must be assessed in further future clinical studies with a longer duration of follow-ups and larger sample sizes.
In addition, from extracted data of our study, one can be concluded that for obtaining the desired recovery from anxiety and depression-related symptoms in comparison with sleep problems, one needs a more vigorous dose or longer duration of melatonin supplementation, or even we can propose that melatonin supplementation firstly improve sleep problems and in consequence, the amelioration in mood changes will be happened by more prolonged duration of melatonin consumption.
However, it is worth mentioning that administration of any agents, including placebo or melatonin, led to the relief of depression and anxiety symptoms, probably due to psychological effects. Our study demonstrated the important role of placebo administration parallel with intended intervention to suppress the placebo-psychologic effects during the studies’ evaluation.
Our study was one of the first pilot studies aiming to assess the impact of melatonin supplementation on attenuating ADT-related psychiatric complications. As it was noted in the results section, we were successful in showing the positive effects of melatonin supplementation on sleep and mood disorders in prostate cancer patients due to ADT. Despite the failure to demonstrate the positive results of melatonin supplementation in reducing psychiatric problems, melatonin improved sleep problems induced by ADT in prostate cancer patients. Large and well-designed studies are suggested to confirm the optimal dose and duration of melatonin supplementation for clinical application.
5.1. Conclusions
The results of the study demonstrated that melatonin supplementation (6 mg daily for four weeks) could improve sleep problems induced by ADT in patients with prostate cancer in terms of sleep quality indices, especially in the sleep latency, disturbance, and duration domains. No statistically significant improvement was reported in the severity of depression and anxiety. Unless non-significant excessive daytime sleepiness, no other significant adverse events were reported. All findings should be examined in large and well-designed studies to confirm the optimal dose and duration of melatonin supplementation.