1. Background
Doxorubicin, an anticancer agent of the anthracycline family, is one of the most popular anti-neoplastic drugs used for decades in the treatment of various cancer tumors, particularly breast and ovarian, leukemia, and lymphoma (1). Nevertheless, doxorubicin (DOX) presents many unfavorable side effects due to its low specificity. Some of these side effects are induced vomiting with nausea, mucositis, myelosuppression, and dose-dependent cardiotoxicity. Cardiotoxicity can result in irreversible heart failure. Also, using the DOX regimen presents acquired multidrug-resistant (MDR) due to several biological mechanisms upon repeated chemotherapy cycles (2, 3).
Multidrug resistance (MDR) is a multifactorial phenomenon and mostly mainly occurs due to various mechanisms with several cellular pathways, for instance, mutations in oncogenes (4) and increased release of the drug outside the cells via ATP-binding cassette (ABC) transporters. P-glycoprotein (P-gp) is a member of the ABC family responsible for carrying DOX out of the cell (5).
Nowadays, to cope with these challenges, controlled drug delivery systems (CDDS) have attracted attention due to improving chemotherapeutics’ performance, including decreasing dose frequency, improving drug efficacy, and reducing side effects (6).
Among various drug delivery systems, organic-based nanocarriers have exhibited great successful systems. These systems are faced with a few disadvantages, such as entrapped drug leakage and premature degradation of the active agent. On the other hand, inorganic material-based nanocarriers can be used as promising alternative drug delivery systems (7).
Since the first discovery of Mobil Composition of Matter No. 41 (MCM-41) materials in 1992 (8, 9), mesoporous silica nanoparticles (MSNs) have been highlighted as a promising alternative drug carrier platform. It can be an efficient drug carrier due to tuneable pore diameter and particle sizes (2 - 10 nm), abundant surface functionalization sites (> 1000 m2/g) (10), superior biocompatibility (11), reproducible synthesis procedure (12), and versatile chemistry for further functionalization (6, 13).
Furthermore, MSNs can accumulate preferably at the tumor site and improve the “enhanced permeability and retention” (EPR) effect. As a result, they have been widely evaluated for cancer diagnosis and therapy and other biomedical applications (14).
Premature drug leakage from nanoparticles in uncoated MSNs could lead to fast dug release (15). To solve these drawbacks, reversible capping was applied to the surface pores of MSNs, improving drug delivery capability. Moreover, improved controlled drug release could be provided by applying one or more stimuli to the cap structure (16).
Stimuli-responsive DDS has developed with the ability to respond quickly to different external (light, ultrasound, magnetic field) or intra-tumoral (redox, pH, enzymes, temperature) initiatives (17). These systems cannot only achieve better control over drug release but also maintain the effective concentration of drugs in targeted tissues for a longer period of time. Temperature is one of the most frequently used factors in stimuli-responsive systems. Body temperature is locally or generally affected by different conditions and environments. Moreover, the temperature is an easily controlled element during various experiments. These attributes make the temperature a convenient factor for designing responsive drug delivery systems (18, 19).
For more than 20 years, numerous researchers have been intrigued by chipping away at the thermo-responsive hydrogels and polymers (20). One of the most studied thermosensitive polymers is poly(N-isopropyl acrylamide) (PNIPAM). It was firstly synthesized in the 1950s (21). Poly(N-isopropylacrylamide) is characterized by an “on” (adhesive) and “off” (non-adhesive) states. It can indirectly change bio-adhesiveness via extending the chain through hydration below the lower critical solution temperature (LCST) of 32°C and collapsing conformation above LCST. These conditions are known as “random coil” and “globular” conformations. Having an LCST nearby human body temperature makes PNIPAM suitable for assembling with MSNs as nanocarriers (18, 22).
The grafting of PNIPAM on the silica nanoparticles can be fabricated by the atom transfer radical polymerization (ATRP) technique. Atom transfer radical polymerization is a living radical polymerization that relies on the equilibrium between dormant and active chains. This method allows narrow molecular weight dispersity of synthesized polymers, predictable chain length, and defined chain-ends (23, 24).
In addition to temperature, redox-responsive behavior is an excellent choice for a better controlled drug delivery. It was demonstrated that various concentrations of glutathione (GSH) are present in different body sites, as it was found at approximately 10 mM in the intracellular matrix and below 0.02 mM in the plasma. Furthermore, in several studies, GSH is located in tumor cells at level 4 orders of magnitude or (at least) higher than normal cells. So, the disulfide bonds are relatively stable in extracellular fluid, whereas they can be cleaved in other environments (25, 26).
Once the drug-loaded MSN-S-S-PNIPAM reached tumor sites, the PNIPAM chain in the outer layer would be collapsed first and then removed to release more DOX after decomposition of the disulfide cross-linker in the presence of glutathione.
2. Objectives
This study aimed to design and construct a chemo-hyperthermia drug delivery platform consisting of thermosensitive PNIPAM as a gatekeeper of release and MSNs as drug containers. The release studies were performed to approve the dual stimuli-responsive character of the designed MSNs. Further studies were conducted to investigate the different characterization of the nanocarriers.
3. Methods
3.1. Material
Doxorubicin hydrochloride (2 mg/mL) was purchased from Korea United Pharm Inc. (Chungnam, Korea). Cetyltrimethylammonium bromide (CTAB), tetraethylorthosilicate (TEOS), (3-glycidyloxypropyl)trimethoxysilane (GPTMS), cystamine, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), 2-bromoisobutyryl bromide (BIBB), dithiothreitol (DTT), ammonium nitrate (NH4NO3), hydrochloric acid (HCl), sodium hydroxide (NaOH), ethanol, triethanolamine (TEA), and chloroform provided by Sigma Aldrich, USA. Deionized water was used for the synthesis purified with the Milli-Q system. All the chemicals were utilized without further purification.
3.2. Methods
3.2.1. Synthesis of Mesoporous Silica Nanoparticles
The surfactant templating method was used to synthesize MSNs according to the Nhavene method (27). Simply, 1.0 g CTAB as the template was dissolved in 480 mL deionized water containing 280 mg NaOH. The mixture was stirred for 15 min at 80°C. Then, 5.36 mL TEOS as a precursor was added drop-wise to the mixed solution under stirring (Heidolph, 400 rpm) for 2 h. The synthesized white precipitate was centrifuged for 15 min at 16000 rpm (Sigma), washed with 50: 50 (v/v) double deionized water and ethanol three times, and freeze-dried for 24 h.
For template extraction, 600 mg NH4NO3 was subsequently added to absolute ethanol solutions of MSN, refluxed for 24 h under an N2 atmosphere, and washed three times with deionized water. Then 5 mL of HCl 37% was added to the MSNs solutions resulting from the previous step under similar conditions.
3.2.2. Functionalization of Mesoporous Silica Nanoparticles with (3-Glycidoxypropyl)trimethoxysilane
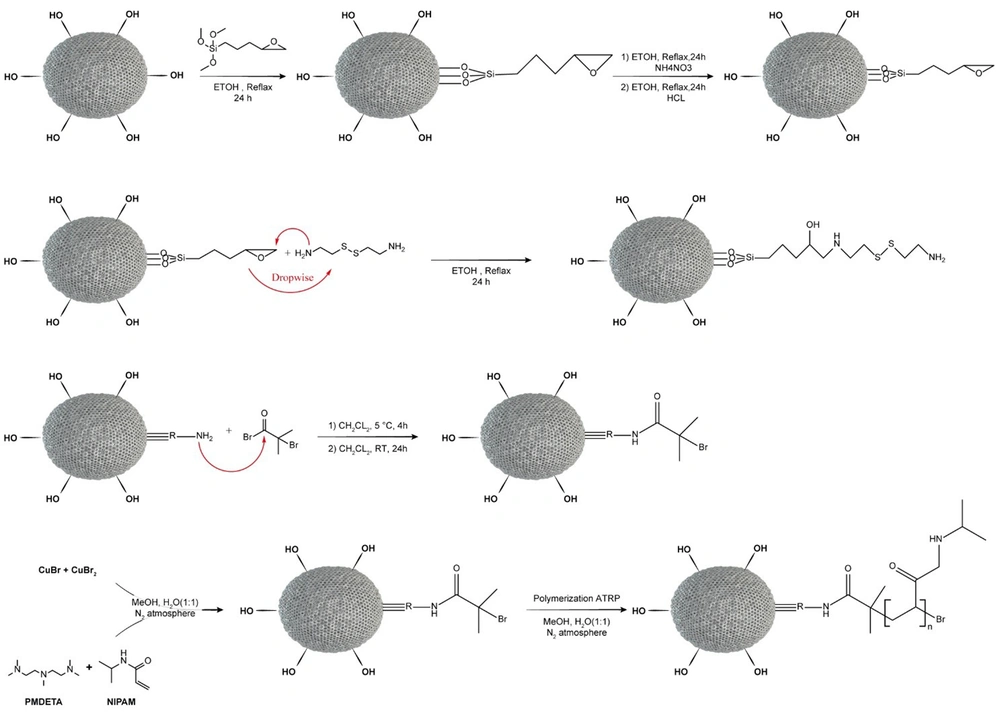
In order to surface modification of MSNs by GPTMS, 700 mg of MSNs was dispersed into absolute ethanol, then 1.5 mL GPTMS (5.5 mM) was added under an N2 atmosphere for 24 h. The process was conducted with the reflux system (Figure 1).
3.2.3. Introduction of Disulfide Cysteamine Groups (Mesoporous Silica Nanoparticle (MSN)-S-S-NH2)
Introducing the disulfide bond onto the MSNs was done via cystamine dihydrochloride. Mesoporous silica nanoparticles-GPTMS and cystamine were dispersed in anhydrous ethanol (the molar ratio is about 2: 1). Afterward, the solution of MSN-GPTMS was added drop-wise to cystamine. The mixture was refluxed for 24 h to form the sulfide band by the reaction of the epoxide group of GPTMS with cystamine.
3.2.4. Producing Atom Transfer Radical Polymerization Initiators on Mesoporous Silica Nanoparticle
The ATRP initiators containing reductive disulfide bonds were prepared by a covalent connection between BIBB and MSN-S-S-NH2. Briefly, MSN-S-S-NH2 was dispersed in dichloromethane, followed by the addition of TEA. 2-bromoisobutyryl bromide dissolved in dichloromethane was added drop-wise to the aforementioned solution in an ice-acetone bath, then permitted the mixture to stir for 4 h at 5°C under N2 atmosphere. The solution was stirred for the next 24 h at room temperature. After centrifuging, washing, and drying, the finished product was obtained (MSN-S-S-Br).
3.2.5. Synthesis of Mesoporous Silica Nanoparticle (MSN)-S-S-Poly(N-isopropylacrylamide) (PNIPAM)
The PNIPAM was covalently anchored to the surface of the MSNs by using MSN-S-S-Br as an initiator (28, 29). A typical procedure is as follows: A ratio of 2.44 g (N-isopropylacrylamide)-co-methacrylic acid (NIPAM), 21 μL of PMDETA as a ligand, and 7 mL of methanol/deionized water (1: 1) were placed into a 20 mL dry vial. Twelve mg CuBr and 16 mg CuBr2 (as catalysts) were placed into another vial. All the pre-sealed systems were exposed to nitrogen gas for 30 min. Subsequently, the monomer-ligand solution was added to a rubber plug sealed vial containing 162 mg MSN-S-S-Br with a glass syringe. The polymerization reaction started with adding the catalysts, and the color of the solution changed to blue. The reaction continued at room temperature with stirring for 24 h. The resulting MSN-S-S-PNIPAM was centrifuged and rinsed repeatedly with ethanol and deionized water.
3.2.6. Physicochemical and Morphological Characterization
The characterization and morphology study of functionalized MSNs were performed using transmission electron microscopy (TEM) images captured from Zeiss Leo 906. The size distribution of nanocarriers was determined by dynamic light scattering (DLS) (Malvern Instruments, Malvern, UK). To examine the long-range order of porous material, X-ray diffraction (XRD) patterns were recorded by Bruker D8FOCUS X-ray diffractometer. Fourier transform infrared spectra (FTIR) were measured in the region of 400 - 4000 cm-1 with a spectrometer (Bruker, Germany) to inspect the structure and major functional groups of MSNs. The chemical composition of samples was analyzed by energy-dispersive X-ray spectroscopy (EDS) analysis.
The thermal stability of nanoparticles was evaluated by thermogravimetric analyses (TGA) using the heating rate of 10°C.min-1 under a nitrogen atmosphere (TGA Q50 V6.3 Build 189).
Ultra-sensitive differential scanning calorimetry (US-DSC) analysis was applied to study phase transition changes of the thermosensitive layer of nanoparticles (0.1% (W/V) MSN-S-S-PNIPAMs aqueous solution) applying a heating rate of 1°C.min-1 from 15°C to 70°C (Micro Cal Inc.).
N2 adsorption-desorption isotherms were evaluated using a surface area and porosity analyzer (BELSORP-miniIIGuo2019).
3.2.7. Drug Loading and In Vitro Release
Drug loading and in vitro release were evaluated by the method described by Huang et al. (16) Briefly, 10 mg of MSN-S-S-PNIPAMs was dispersed in 20 mL PBS (pH = 7.4). One milliliter of various aqueous concentrations of DOX (5, 10, 15, and 20 mg/mL) was prepared and stirred with the nanocarriers at 25°C for 24 h in a dark place.
Finally, after the loaded nanoparticles of DOX-MSN-S-S-PNIPAM were separated with a magnet, the product was rinsed several times with deionized water to remove the unbound drugs. The content of free DOX was estimated using the UV absorbance of the supernatants measured at 490 nm using the calibration curve. The drug loading content (DL) and the drug encapsulation efficacy (EE) were determined as follows (16):
The release behavior of DOX was performed in the presence or absence of DTT (20 mM) using a dialysis bag (12 kDa molecular weight cut-off). Besides, to obtain the phase transition temperature of the MSN-S-S-PNIPAM, the experiment was carried out at 25°C and 41°C.
Ten milligrams of DOX-loaded nanoparticles were suspended in 20 mL of phosphate-buffered saline (PBS, 40 mM, pH = 7.4) and set in a dialysis bag with stirring at 90 rpm. Three milliliters of release medium were taken at defined time intervals and replaced with equal fresh pre-warmed PBS. The concentration of DOX was determined by UV measurement, as mentioned before. Each experiment was performed in triplicate, and the mean ± SD of the results was reported.
3.2.8. In Vitro Cytotoxicity Assays
Nanoparticle cytotoxicity studies on Michigan Cancer Foundation-7 (MCF-7) cells were assessed by the MTT test. Michigan Cancer Foundation-7 cells were cultured in 96-well plates (1 × 104 cells/well) for 24 h in a 5% CO2 humidified atmosphere. After 24 h, the old medium of each well was replaced with the fresh medium containing free DOX or DOX-loaded nanocarriers with the same concentrations. After incubation for 48 h, 20 μL of MTT (5 mg/mL, PBS) solution was added, and the cells were further incubated at 37°C for 4 h. The medium was sucked out, and 150 μL of dimethyl sulfoxide (DMSO) was added to each well. After mixing, the optical density values of the samples are measured at 570 nm (30).
4. Results and Discussion
4.1. Design and Preparation of Mesoporous Silica Nanoparticle (MSN)-S-S-Poly(N-isopropylacrylamide) (PNIPAM)
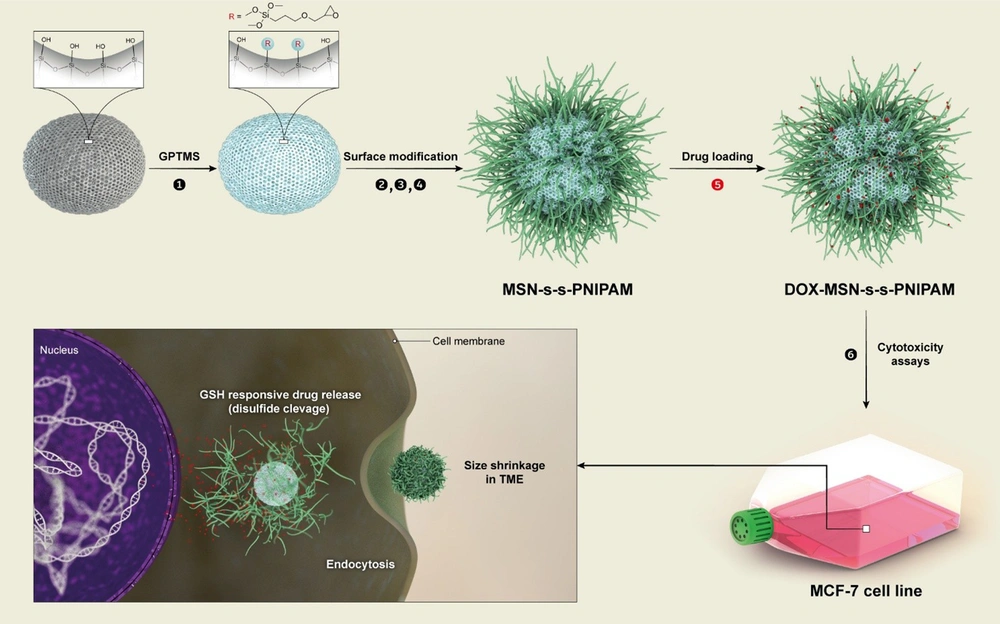
Like many other chemotherapeutic agents, low permeability (DOX class III biopharmaceutical classification system (BCS)) and acute systemic toxicity have limited the efficacy and usage of DOX. To overcome these disadvantages and early release of DOX, DOX was loaded to MSNs and linked to thermosensitive polymer and disulfide bands to control the release via temperature, and redox dual modulated responsive system. As proof of concept, the synthetic process of MSN-S-S-PNIPAM is shown in Figure 2. Mobil Composition of Matter No. 41 was firstly synthesized by the surfactant templating method.
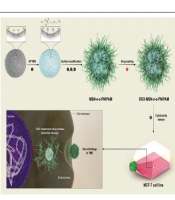
A schematic pattern of synthesizing stimuli-responsive mesoporous silica and cellular uptake and doxorubicin (DOX) release of mesoporous silica nanoparticle (MSN)-S-S-poly(N-isopropylacrylamide) (PNIPAM). (1) (3-glycidyloxypropyl)trimethoxysilane (GPTMS); (2) cystamine; (3) 2-bromoisobutyryl bromide (BIBB); (4) (N-isopropylacrylamide)-co-methacrylic acid (NIPAM)/hydroxy ethyl methacrylate (HEMA); (5) DOX-loading at low temperature; (6) cytotoxicity assays
In this method, TEOS was utilized as a silicon source, and CTAB, a cationic surfactant, was utilized as a template to fabricate pore structures in an alkaloid environment. In order to prevent size changes by NaOH, the final product was washed with deionized water and ethanol. Then, the process of removing the template was done. Compared with other methods of removing CTAB, the previously mentioned method did not increase the particle size of MSNs.
Afterward, to get the reductive MSN-S-S-NH2, MSNs were functionalized with GPTMS, and the MSNs-GPTMS reacted with cystamine.
To achieve dual responsive targeted drug release, the MSN-S-S-NH2 was covalently bound to NIPAM polymer via ATRP technique. 2-bromoisobutyryl bromide was used as an ATRP initiator. The presence of the –Br group in the EDS has been performed by the BIBB binding to MSN-S-S-NH2. The synthesis steps of MSN-S-S-PNIPAM are presented in Figure 1.
Energy-dispersive X-ray spectroscopy, FTIR, and TEM analyses were done to confirm whether each step modification on MSNs was rightly completed. Finally, the smart delivery system is produced after loading DOX into the nanocarriers by physical adsorption.
4.2. Morphology and Size Analysis of Nanoparticles
4.2.1. Fourier Transform Infrared Spectra Analysis
Figure 3 shows the FTIR spectra of different steps of the prepared nanoparticles. The strong peak around 1088 cm-1 was attributed to the stretching of the Si–O–Si band. The presence of two sharp peaks at 2859 and 2974 cm-1 corresponds to the C–H stretching band of the CTAB. After the removal of the surfactant, the peaks disappeared. After the modification of MSNs with cystamine, the peaks around ~1346 cm-1 were implied to stretching of C-N bonds, whereas the peaks at 1474 cm-1 and 1554 cm-1 were shown as the N-H bands of the NH2 group.
Vibration bands at 1657 and 1589 cm-1 are correlated to amide Ι and amide ΙΙ bands (N-H stretching). Also, in 1492 cm-1, the amide III bands can be distinguished. Furthermore, the peaks about 1380 and 2974 cm-1 raised due to –CH(CH3)2 and –CH3. All these data indicate the successful attaching of PNIPAM polymer to the surface of mesoporous silica nanoparticles.
Zeta potential measurements evaluated the surface charge of prepared samples. The zeta potential of primary MSNs was about +25 mV. After the addition of GPTMS, the zeta potential changed to +16 mV because of decreasing in hydroxyl groups of the MSN surface. The zeta potential altered to -20 mV after the CTAB removal process. The zeta potential of modified MSN with cystamine returned to highly positive voltage again (around +20 mV), which could be due to the presence of –NH2.
4.2.2. Energy-Dispersive X-Ray Spectroscopy Analysis
Energy-dispersive X-ray spectroscopy analysis was used for the elemental characterization of the samples at each step of modifications. To investigate the complete removal of the CTAB, EDS analysis was conducted, and the result showed only the silicon and oxygen peaks, as similar outcomes obtained by FTIR.
After the modification with cystamine, the EDS result contains 1.04% nitrogen, 5.38% carbon, and 0.98% sulfur in addition to oxygen and silicon. Furthermore, the bromine peak at MSN-BIBB analysis confirmed the successful attachment of –Br to the mesoporous nanoparticles.
4.2.3. X-Ray Diffraction Analysis
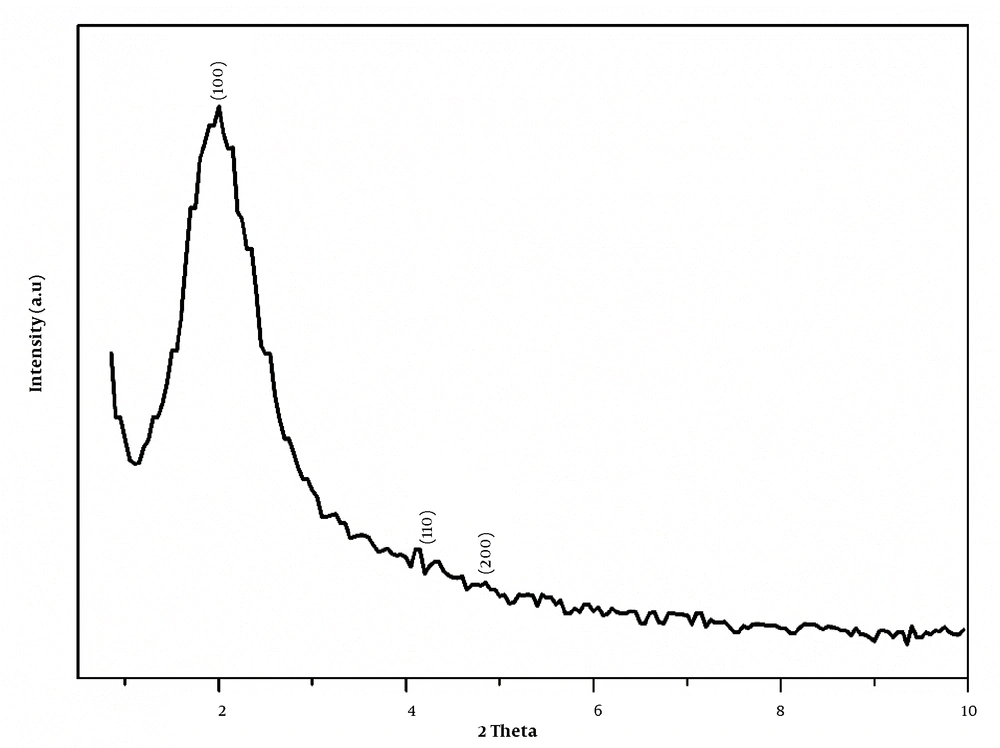
The XRD pattern of MSN is depicted in Figure 4. As described before, the synthesized mesoporous silica for using CTAB is MCM-41. The XRD pattern (1 0 0), (1 1 0), and (2 0 0) proved to prepare a highly ordered hexagonal structure of mesoporous silica structure (31).
4.2.4. Nitrogen Adsorption/Desorption
In order to evaluate nanoparticles porosity and surface specification, the parameters, including Brunauer-Emmett-Teller (BET) surface area (SBET), mean pore diameter (dp), and cumulative pore volume (Vp) of the MSN and MSN-S-S-PNIPAM were measured by the nitrogen adsorption-desorption method (32).
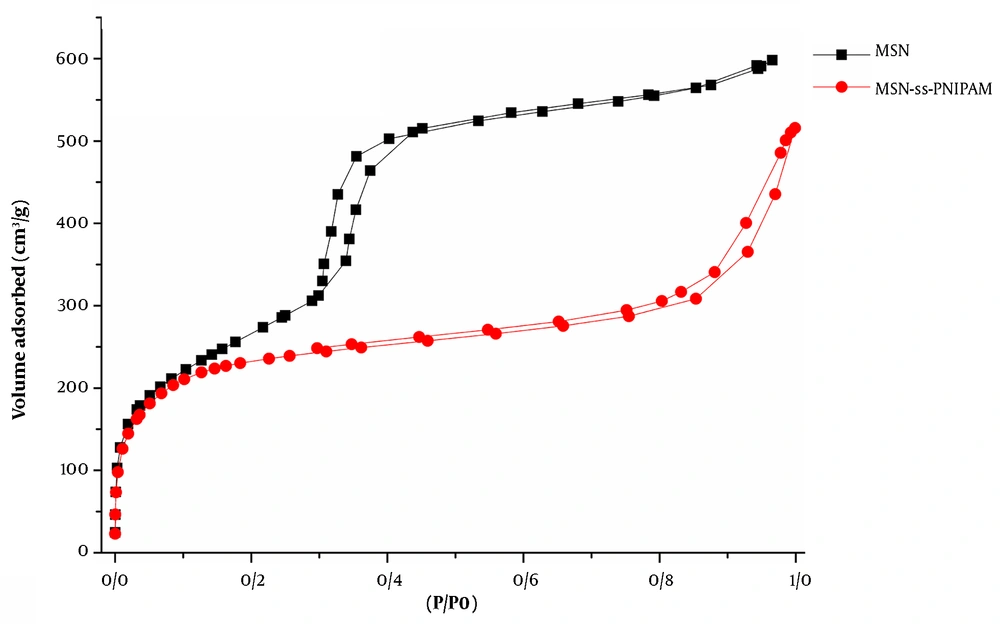
Mesoporous silica nanoparticles present the typical type IV curve based on the International Union of Pure and Applied Chemistry (IUPAC) nomenclature (Figure 5). In the isotherm of naked MSNs, a well-defined H1-type hysteresis loop with a sharp capillary condensation step at P/P0 of 0.3 - 0.6 shows a typical characteristic of well-ordered mesoporous compounds with cylindrical channels. It can be concluded that after all the modification steps, the nature of mesoporous nanoparticles is preserved well (33).
As depicted in Figure 5, MSN-S-S-PNIPAM adsorbed lesser gas volume, affecting the lower level of the hysteresis loop. Hence, the nanoparticles are partially occupied by the polymer and still have sufficient space for drug loading.
Brunauer-Emmett-Teller surface area, Vp, and dp of MCM-41 were 939 m2g-1, 0.94 cm3g-1, and 2.4 nm. After capping the MSNs with thermosensitive polymers, these factors decreased dramatically to 596 m2g-1, 0.68 cm3g-1, and 2.1 nm. This outcome suggests that PNIPAM can be found in the interior pores and the exterior surface.
4.2.5. Thermogravimetric Analysis and Differential Scanning Calorimetry Analysis
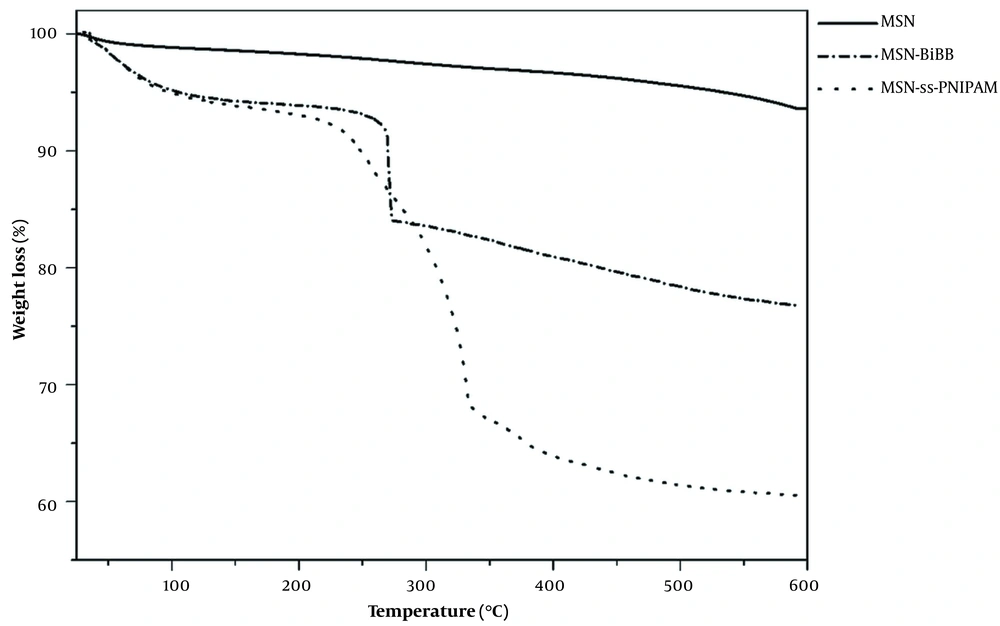
Figure 6 revealed the TGA profiles of MSN, MSN-BIBB, and MSN-S-S-PNIPAM. The TGA results verified the successful modification of MSNs with PNIPAM. Samples were heated to 600°C under the N2 atmosphere. In the TGA curve of MSN, 3% weight loss occurred at a temperature under 150°C, related to the removal of physically adsorbed water. In the thermogram of MSN-BIBB and MSN-S-S-PNIPAM, the removing adsorbed water was observed in the range of 25 - 100°C. In the curve of MSN-BIBB, the grafted functional groups’ degradation was observed in the region from 250 to 300°C. Compared to MSN-BIBB, the extra weight loss of MSN-S-S-PNIPAM occurred at around ~330°C; (about 20% of total weight), which is attributed to the decomposition of the thermosensitive polymer. This mass reduction was considered equivalent to the coated PNIPAM shell.
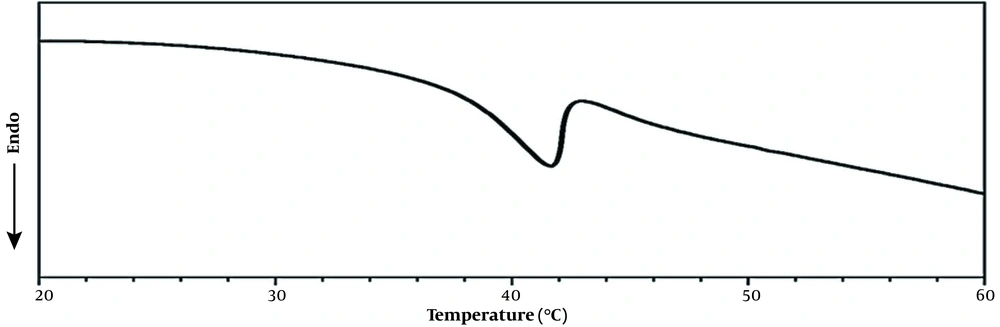
Differential scanning calorimetry test of the MSN-S-S-PNIPAM solution was carried out in deionized water to evaluate the phase transition behavior of the PNIPAM shell (Figure 7). It was observed that the heating curve of PNIPAM attached MSNs showed a phase-transition temperature at 39°C that was more than the LCST of pure PNIPAM. Compared to the LCST of neat PNIPAM (about 32°C), the endothermic peak values of PNIPAM might be affected by the HEMA monomer in the structure of the thermo-responsive shell.
4.2.6. Transmission Electron Microscopy Analysis
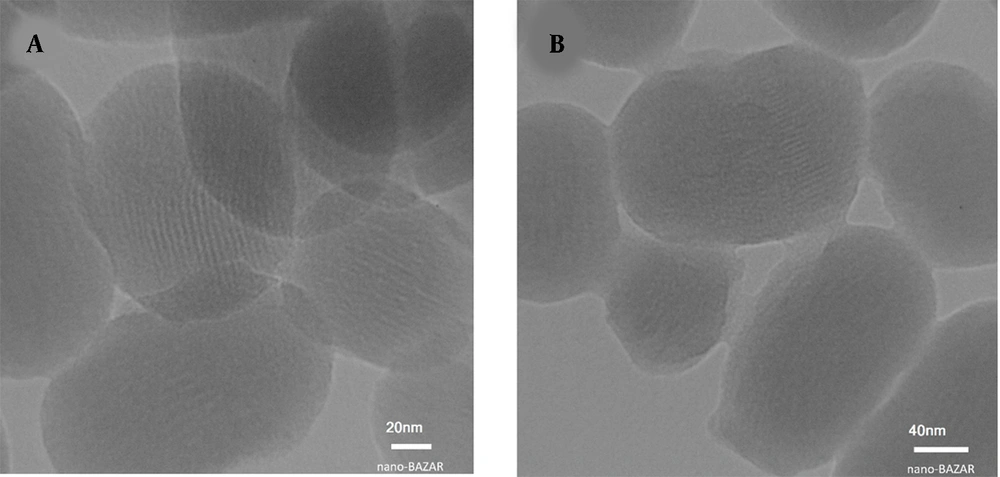
Transmission electron microscopy images of Figure 8 illustrated a uniform and spherical shape (toward bean-shaped) of mesoporous nanoparticles with an average size of around 150 nm. The MSNs consist of a series of hexagonal parallel channels. Moreover, in Figure 8A, the mesoporous structure with a pore diameter of 2.5 nm can be clearly distinguished. In the TEM image of MSN-S-S-PNIPAM, the core-shell structure is clearly seen due to the presence of the PNIPAM layer. The distinct grey shell-like layer has a 3 - 6 nm diameter. Also, based on the pictures, the structure of MSNs did not decompose and retain the original size and diameter.
4.2.7. In Vitro Drug Loading and Release Profile
The drug loading content (DLC) and EE of DOX into MSNs carriers were investigated by the UV absorbance method (Table 1).
| Doxorubicin: Polymer Ratio | Encapsulation Efficiency (%) | Drug Loading Content (%) |
|---|---|---|
| 0.5: 1 | 48 | 24 |
| 1: 1 | 35.4 | 36 |
| 1.5: 1 | 29.5 | 42 |
| 2: 1 | 22 | 44 |
By increasing DOX to nanoparticle polymer-coated ratio from 0.5: 1 to 2: 1, the DLC % of MSNs increased from 24% to 44%, while the EE decreased from 48% to 22% due to the saturation adsorption of MSNs with the drug molecules. Considering the amount of DOX waste, the optimum drug: Nanoparticle ratio was found to be 1.5: 1. At this ratio, the average drug loading content and encapsulation efficacy were reported as 42% and 29.5%.
4.2.8. Release Study
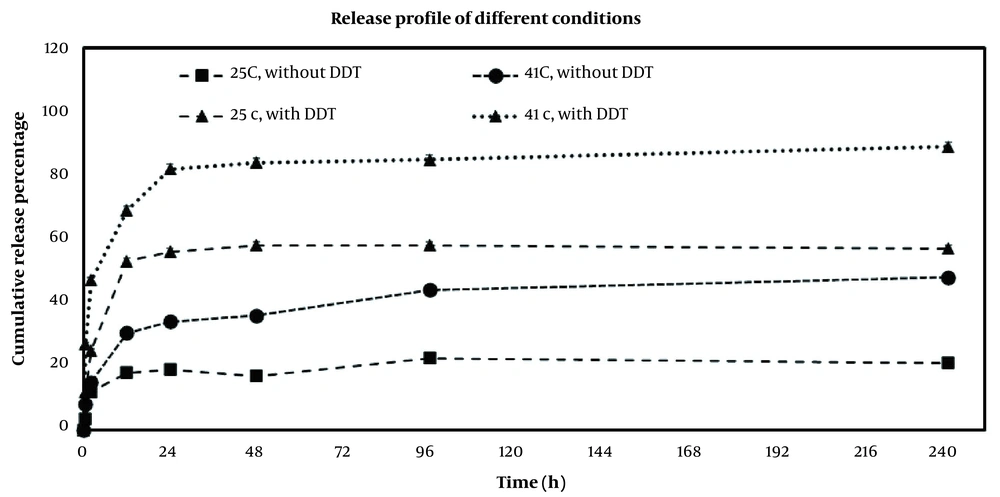
As shown in Figure 9, the drug release profiles of DOX-MSN-S-S-PNIPAM with or without the addition of DTT at different temperatures were measured. At first glance, it is obvious that both temperature and DTT have a synergistic impact on controlling DOX release from the nanoparticles, confirming the double responsive release of mesoporous nanoparticles. However, these dual stimuli-responsive MSNs show a stronger release rate dependence on temperature (i.e., a more significant difference between 25°C and 41°C).
The initial burst release of the drug occurred within 2 h, which 12% and 15% of DOX were released at 25°C and 41°C with the absence of DTT in release media, while these numbers decreased to 25% and 47% at 25°C and 41 °C in the presence of DTT. The physical adsorption of the DOX molecules might have an important role in the burst release.
During 12 h, the cumulative release amount of DOX was 30.5% at 41°C and 18% at 25°C in the absence of DTT. Adding 20 mM of DTT accelerated the release of DOX to 69% at 41°C and 53% at 25°C.
As mentioned in the introduction, PNIPAM, a well-known thermosensitive polymer, acts as a gatekeeper of drug transmission. Hence, below LCST, the long chains of PNIPAM stretch because of the formation of hydrogen bonds between polymer and water, so the polymers inhibit the drug release. Above its LCST, polymers start to shrink and dehydrate, which could lead to decreasing the gap between polymer chains and shorten the release path, so the entrapped DOX could be quickly released (34). The DOX release also depends on the redox process. According to reports, the existence of glutathione plays a key role in the redox targeted delivery due to its higher concentration in cancer cells (35). The DTT attendance in the release medium is similar to the presence of glutathione. The disulfide bonds were broken down by DTT, and the PNIPAM was detached; thus, the channels of MSNs were opened, and the release of DOX molecules was accelerated remarkably.
4.2.9. In Vitro Cytotoxicity Assays
Different cell models derived from mammalian cells are used as a suitable method to analyze the biocompatibility and cytotoxicity of chemotherapeutic agent nano-formulations. Michigan Cancer Foundation-7 cells, one of the breast cancer cell lines used as a cell model to determine their cell viability and proliferation against different samples of both groups (free DOX and DOX loaded NPs). This assay was measured by using tetrazolium salt MTT (30).
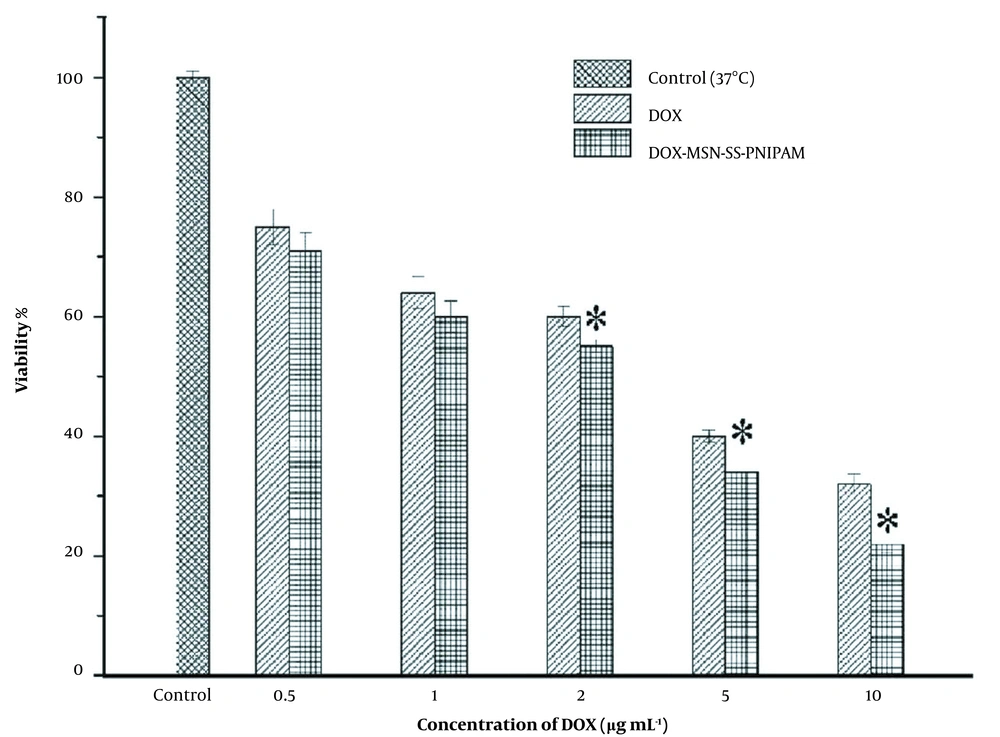
Generally, the increment of the DOX concentrations results in the cytotoxicity elevation of both formulations. A significant reduction in cell population was observed in the MCF-7 cells exposed to DOX-MSN-S-S-PNIPAM compared to free DOX. The difference between the viability of the cells treated with nano-formulations and the free drug increased as the drug concentration increased.
Many researchers tried to explain these differences as a result of the cellular uptake mechanisms of the drug. Free DOX can easily pass through the cell membrane and enter its active site. As mentioned, DOX molecules were pumped out of the cells via P-gp systems, leading to reduced chemotherapy efficacy. On the other hand, the internalization of DOX-loaded nanoparticles into cells occurs through passive endocytosis. Thus, the cellular uptake of DOX was enhanced notably (36).
Furthermore, as Figure 10 shows, the cell viability of MCF-7 cells in 0.5 μg.mL-1 was about ~71% in DOX loaded MSN-S-S-PNIPAM and ~75% in free DOX, while in 10 μg.mL-1 it was ~22% and ~32%, respectively.
The cytotoxicity of doxorubicin (DOX) and DOX-mesoporous silica nanoparticle (MSN)-S-S-poly(N-isopropylacrylamide) (PNIPAM) in different concentrations against Michigan Cancer Foundation-7 (MCF-7) cell line. Results were expressed as mean ± SD (n = 5); significance was calculated by ANOVA (* P ≤ 0.05).
So it can be concluded that at the range of 0.5 - 10 μg.mL-1, both DOX and DOX loaded MSN-S-S-PNIPAM illustrated concentration-dependent toxicity against cells.
All data were presented as mean ± SD. Parametric variables were analyzed using a one-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. All statistical analyses were performed in SPSS 18 (SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered significant for all statistical tests.
4.3. Conclusions
The nanoparticles were used for the controlled release of DOX to cancer cells.
In this study, thermosensitive PNIPAM chains grafted to the mesoporous silica, a stimuli-responsive drug delivery platform, were developed. In addition, the nanoparticles, including disulfide bonds, were used for grafting the polymeric nanoparticles to have a dual-responsive system.
Nanoparticles, including a grey shell-like layer with a diameter of 3-6 nm on the surface, were developed by grafting NIPAM on MSN.
To find the best condition for drug loading and encapsulation of DOX into MSNs carriers, different concentrations of the DOX were used. In optimum conditions, with a ratio of 1.5: 1 of DOX to the nanoparticle, the DLC% was 42%, while the EE was 29.5%. In comparison, the lowest EE was achieved with a ratio of 2: 1.
To evaluate DOX-MSN-S-S-PNIPAM nanoparticles’ responsibility, the drug release profiles in the presence and absence of DTT at 25°C and 41°C were evaluated. Doxorubicin release was 63% more at 41°C and the presence of DTT, in comparison to 25°C and absence of DTT at 24 h, confirmed the double responsive release of mesoporous nanoparticles. These results showed that DOX-MSN-S-S-PNIPAM nanoparticles are dual stimuli-responsive.
According to the MTT test results, these controlled release DOX-MSN-S-S-PNIPAM nanoparticles have shown more efficient cytotoxicity compared to DOX against the MCF-7 cell line. This effect may be due to the cell uptake of MSN-S-S-PNIPAM, which should be evaluated by further studies. Regarding the beneficial behavior of the nanoparticles, it could be concluded that MSN-S-S-PNIPAM could be an interesting candidate for the smart delivery of DOX to cancer cells.