1. Background
Curcumin, a safe and well-known natural compound, is derived from the rhizomes of the Curcuma longa and is used as a nutritional supplement. Curcumin affects cellular pathways, and its role has been reported to induce cell differentiation (1, 2). The compound regulates many proliferative and oncogenic factors and chemo-resistance associated genes or proteins, representing the anticancer role (3). However, low solubility and poor cellular uptake of curcumin have limited its application (4, 5). Therefore, promising methods have been developed to increase its solubility using polymeric nanoparticles such as dendrosomes as low-cost, non-toxic, neutral, and biodegradable agents. In this regard, oleic acid-derived dendrosome (OA400) with a 200 nm diameter, polydispersity index (PDI) of 0.4, z-potential of seven, and high loading efficiency (87%) has been used to increase the solubility and physical and chemical stability of curcumin and has also demonstrated its significant anti-tumor properties in both in vitro and in vivo studies (6-10).
Cervical cancer is the third most common cancer in women. Five hundred sixty-nine thousand new cases and 311,000 deaths from this cancer were reported in 2018 (11). Central risk factors for cervical cancer include human papillomavirus (HPV), smoking, and immune disorders (12). Carcinogenic HPV types (such as HPV16 and HPV18) target cervical epithelial cells and are responsible for almost all cases of cervical cancer (13). Among the genes encoding HPV (14), E6 and E7 are the primary oncogenic factors that target different cellular proteins. E6 protein inactivates the P53 tumor suppressor by binding to it and eliminates the sensitivity of cells to checkpoint and apoptosis signals (15). E7 protein can disrupt the Rb, terminates their association with the E2F transcription factor family, and destroys cell cycle regularity (16). Moreover, E6 and E7 genes’ expression increases in the full-thickness epithelial lesion in cervical cancer (17-19).
The effect of curcumin on cervical cancer cells and its anti-HIV property have been investigated (20). Curcumin can arrest the proliferation of cervical cancer cells depending on the time and concentration, and it is more active in HPV-infected cells. The anticancer activity of curcumin against cervical cells was due to the regulation of telomerase activity, Ras and ERK signaling pathways, cyclin D1, COX-2, and iNOS activity, and mitochondrial pathway. Recent proteomic studies have shown that curcumin causes significant changes in proteins associated with cell metabolism, cell cycle, and carcinogenesis in HeLa cells. The decrease in cervical tumor volume has also been demonstrated in the curcumin-treated mouse model (21).
2. Objectives
Considering the importance of cervical cancer, this study examined the effect of nano-curcumin on the expression of E6, E7, Rb, and P53 in the RNA and protein levels of HeLa cancer cell lines. Furthermore, the cell viability reduction and apoptosis induction were determined in HeLa cells treated with curcumin-loaded OA400 nanoparticles.
3. Methods
3.1. Nanocurcumin Preparation
PEGylated dendrosomes as nano-carriers were prepared according to the previously optimized protocol (7). Briefly, an esterification reaction of oleoyl chloride (0.01 mol) and polyethylene glycol 400 (0.01 mol) in the presence of triethylamine (0.012 mmol) and chloroform was performed for the synthesis of OA400. The reaction was accomplished at 25°C for four hours. Triethylamine hydrochloride and chloroform were removed by filtration and evaporation in a vacuum oven (40°C for four hours). Curcumin and the prepared polymeric carriers were mixed using different weight-weight ratios (from 1: 10 to 1: 50) and incubated overnight at 37°C. Then, absorbance spectra were evaluated via ultraviolet spectrophotometry (Infinite 200 PRO, Tecan, Mannedorf, Switzerland).
The best mixture of curcumin and nano-carrier was determined using spectrophotometry at 420 nm wavelength. The weight-weight ratio of 1: 25 was obtained as a suitable and stable ratio (without curcumin precipitation from the nano-carrier). Curcumin and dendrosomal carriers were then mixed with this optimal ratio in acetone. After evaporation of acetone, the curcumin/dendrosome solution was sterilized using a 0.22 mm syringe filter (EMD Millipore). The prepared nano-curcumin was stored at 4°C and away from light (7, 8).
3.2. Cell Culture and Reagents
The human cervical cancer cell line (HeLa cell line RRID: CVCL_003) and normal fibroblast cells were purchased from the Pasteur Institute of Iran. DMEM growth medium containing 10% (v: v) fetal bovine serum (FBS) and 1% (v: v) of penicillin and streptomycin (all from GibcoBioCult, Paisley, Scotland, UK) was used for the culture of HeLa and fibroblast cells. Incubation condition consisted of CO2 (5%) at 37°C. Hemocytometer and trypan blue staining defined cell number and viabilities. These cells were passaged via trypsin/EDTA (biosera, Franc).
3.3. 3-(4, 5- Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT) Assay
The effects of nano-curcumin, curcumin, and OA400 on HeLa and fibroblast cells viability were detected via 3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. 3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide colorimetric assay is a method that determines differences between viable cells and necrotic ones (22).
Briefly, the number of cells was measured by a hemocytometer using trypan blue staining. Approximately 104 cells were seeded in each well of 96 well-plate. After incubation for 24 hours, cellular monolayers were formed, then treatment with nano-curcumin (10 μM - 40 μM), curcumin (10 μM - 70 μM), and OA400 (10 μM - 160 μM) was performed for 48 hours. Since cancer cells are more sensitive to curcumin than normal cells (23), fibroblast cells were treated with higher concentrations of this compound. Then, the medium was replaced with a solution containing MTT (0.5 mg.mL-1) (Sigma Aldrich Company) and incubated for four hours at 37°C in 5% CO2. After gently removing the supernatant, Formazan crystals were dissolved in 200 μL of dimethyl sulfoxide (DMSO from Sigma Aldrich Company), and the optical density (OD) of the samples was measured at 540 nm by a plate Reader (BioTek Company, USA). Cell survival percentage was obtained by the absorption ratio of the treated cells to the control cells. Inhibitory concentration (IC50) was the concentration at which 50% of the cells were destroyed.
3.4. Cellular Apoptosis Analysis via Flow Cytometry
The apoptosis assay treated HeLa and fibroblast cells with curcumin (50 μM) and nano-curcumin (15 μM) according to the IC50 value. After 48 hours, the cells were stained with annexin V/PI (propidium iodide) apoptosis detection kit according to the manufacturer’s instruction (Ebioscience, Thermo Fisher Company). Cell apoptotic and necrosis levels were detected via flow cytometry (FACS Calibur, USA). FlowJo software (version10, Treestar, USA) was subsequently qualified for data.
3.5. RNA Extraction, cDNA Synthesis, and Real-Time RT-PCR
Total RNA was extracted from treated and untreated HeLa and fibroblast cells with curcumin and nano-curcumin using TRIzol reagent (Invitrogen, Cat.no: 15596-026). Extraction steps were performed according to the instructions of the Favorgen Biotech kit (Favorgen Biotech Company, Taiwan), and extracted RNAs were qualified by UV spectrophotometry.
RNA was reverse transcribed using a cDNA synthesis kit (Favorgen Biotech Company, Taiwan). Treatment with DNase I enzyme was performed to ensure that RNA was not contaminated with DNA.
Quantitative measurement of E6, E7, P53, and Rb gene expression was performed using the comparative quantification method by real-time RT-PCR in ABI Step One Sequence Detection System (Applied Bio-systems, CA, USA). In this method, SYBR® Premix Ex Taq™ II (TAKARA, Japan; Cat.RR82LR) was applied according to its manufacture, and the GAPDH gene was utilized as an internal control. Specific primer sequences of target genes are shown in Table 1, and their concentrations were 10 pM in the reaction. The 2-ΔΔCt method was utilized to calculate the relative expression of genes.
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| E7 | 5’-CAATTAAGCGACTCAGAGGAAG-3’ | 5’-ACCACGGACACACAAAGG-3’ |
| Rb | 5’-CAGATGAAGCAGATGGAAGTAAA-3’ | 5’-AGAGACAATGAATCCAGAGGTG-3’ |
| E6 | 5’-GCGACCCTACAAGCTACCTGAT-3’ | 5’-GCACCGCAGGCACCTTATTA -3’ |
| P53 | 5’-GCCGCAGTCAGATCCTA-3’ | 5’-CTGGGAGCTTCATCTGGA-3’ |
| GAPDH | 5’-CCGAGCCACATCGCACAG-3’ | 5’-GGCAACAATATCCACTTTACCAG-3’ |
3.6. Western Blotting Analysis
The E7, P53, and Rb protein levels in curcumin (15 μM) and nano-curcumin (15 μM) treated HeLa cells and the control group was evaluated using Western blotting. For protein extraction, the cells were lysed in RIPA buffer (Cell Signaling Technology, Cat No: 9803). This buffer contains 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM EGTA, 1% NP-40, and 1% sodium deoxycholate. Quantitation of extracted protein was determined by Bradford staining and flow cytometry. Equal volumes of protein samples (40 μg) were disrupted at 90°C for 5 min, subjected to SDS-PAGE, and transferred to polyvinylidene difluoride (PVDF) filter membranes. The bovine serum albumin (BSA)-blocked membranes were incubated with specific primary antibodies for E7, P53, and Rb proteins (Mouse monoclonal antibodies, prepared from Royan Institute of Iran) at 4°C overnight according to manufacturer instructions. Horseradish peroxidase-conjugated secondary antibody (HRP Rabbit Anti-Mouse (IgG) secondary antibody purchased from Abcam Company) was employed to attach to primary antibodies with 1: 1000 v: v concentration. Protein bands were detected with the enhanced chemiluminescence (ECL) method (Biomedical Company). The internal control for this experiment was GAPDH protein. This protein was detected by mouse GAPDH primary antibody (1: 1000, Abcam Company) and antimouse HRC conjugated secondary antibody (1: 1000) (from Santa Cruz). Western blot results were qualified via Image j software (version 1.2.4 RRID: SCR_003070), and the significance of the results was indicated by GraphPad Prism (version 7 RRID: SCR_002798).
3.7. Statistical Analyses
Statistical analyses were performed by GraphPad Prism 7 software. Student’s t-test was used for deference examination between two groups. Probability values were set at the significant levels of P < 0.05 (P < 0.05 (*), P < 0.01 (**), P < 0.001 (***) and P < 0.0001 (****).
4. Results
4.1. Curcumin, Nanocurcumin, and Nanocarrier Effect on Hela and Fibroblast Cells Viability
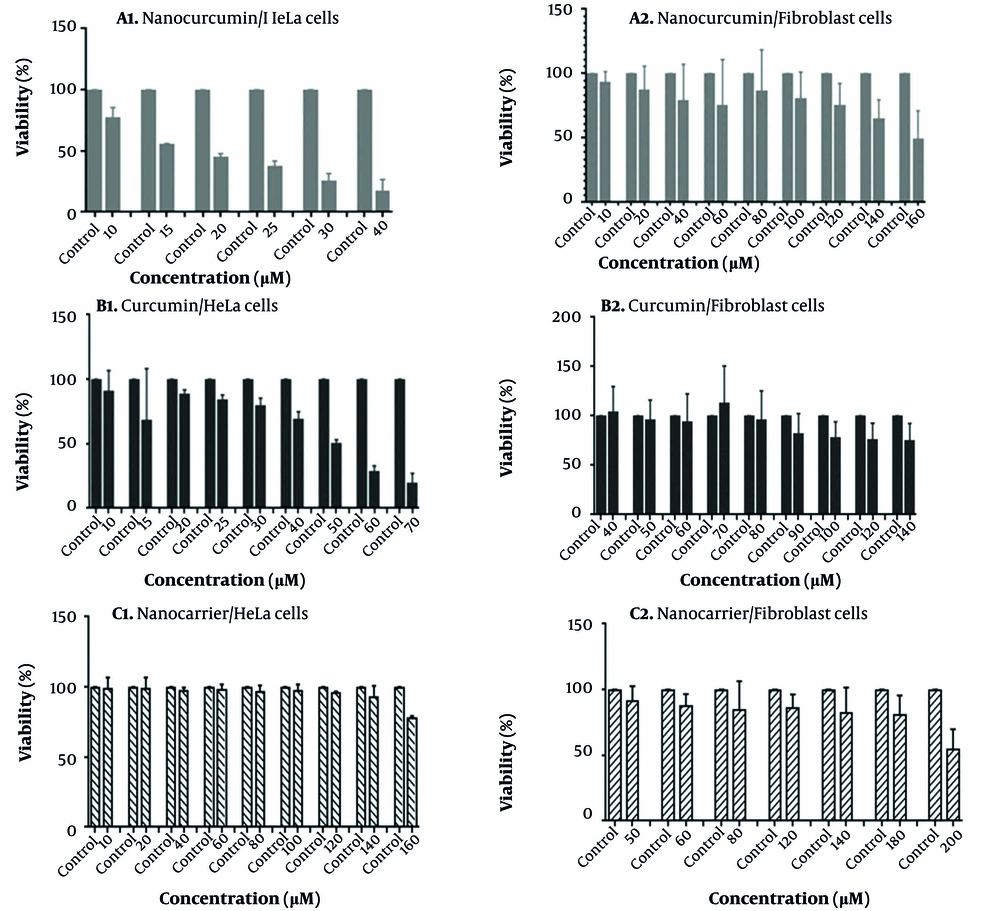
3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide assay results of nano-curcumin, curcumin, and nano-carrier treatments with different concentrations on the survival of the HeLa cell line are shown in Figure 1. As shown in this figure, the increasing concentrations of nano-curcumin (10 - 40 μM) in the media in the cancerous HeLa cell line can significantly decrease the percentage of viable cells after 48 hours. The IC50 value of nano-curcumin for the HeLa cell line within 48 hours was about 15 μM (Figure 1A1). This growth inhibitory effect of curcumin is much less than nano-curcumin. The value of IC50 for HeLa cells treated with curcumin for 48 hours has been increased to about 50 μM (Figure 1B1). Oleic acid-derived dendrosome nano-carrier has no inhibitory effect on HeLa cell growth. In this experiment, cells were treated with nano-carrier up to 160 μM, but at none of the concentrations, cell survival reached 50% (Figure 1C1).
3-(4, 5- dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay to evaluate cell viability; MTT assay result of HeLa cells treated with A1, Nanocurcumin; B1, Curcumin; and C1, Nanocarrier, and fibroblast cells treated with A2, Nanocurcumin; B2, curcumin; and C2, Nanocarrier. Error bar lines indicate the standard deviation level of samples. The control samples include cells that have not been treated.
Interestingly, nano-curcumin has a non-significant effect on the growth of normal fibroblast cells. The IC50 value was observed at very high concentrations (about 160 μM, Figure 1A2). Pure curcumin and nano-carrier also had no significant inhibitory effect on fibroblast cell growth (Figure 1B2 and C2).
4.2. Nanocurcumin Effect on Apoptosis in HeLa and Fibroblast Cells
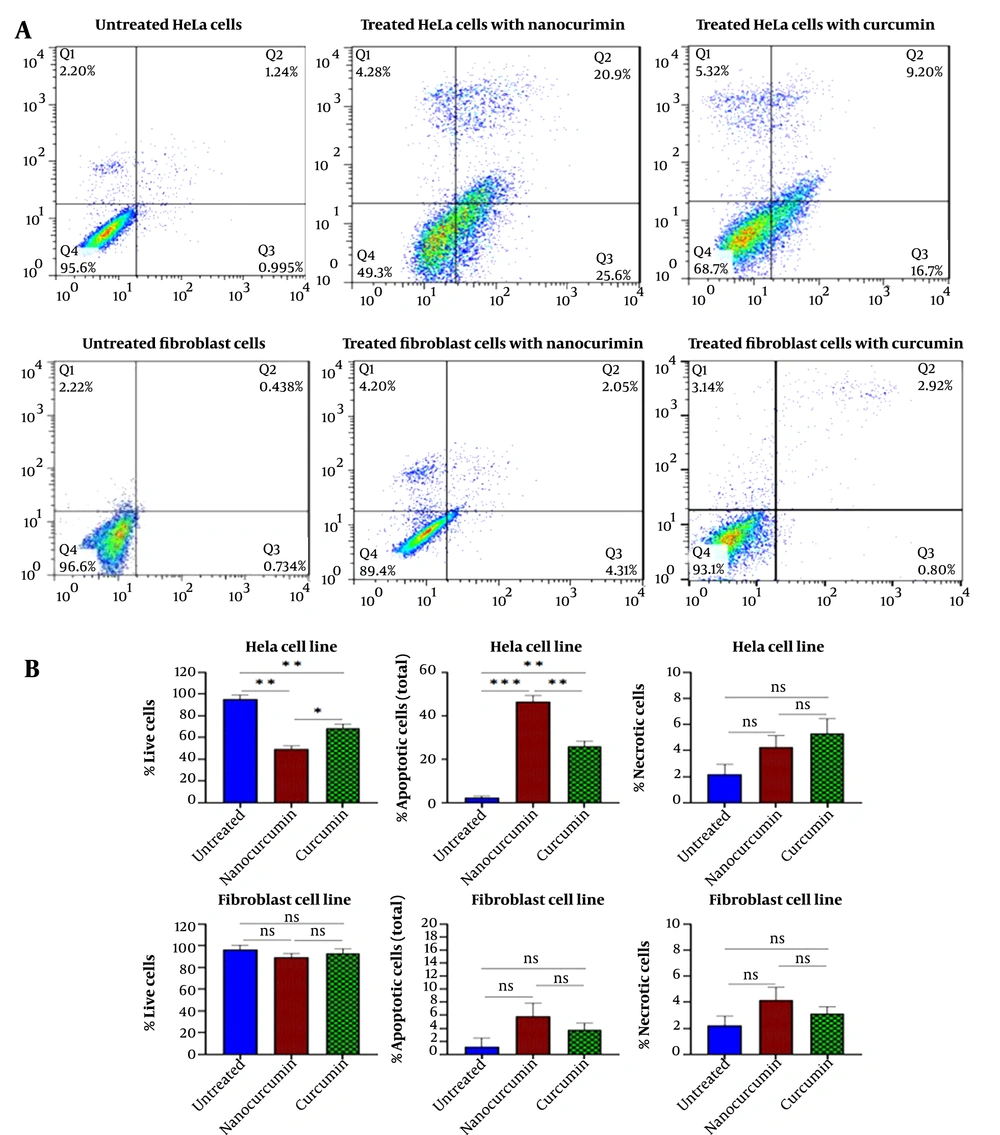
Anxin-PI staining and flow cytometry were accomplished to evaluate the effect of nano-curcumin and curcumin on the apoptosis of HeLa and fibroblast cells. Flow cytometry results showed that 46.5% of HeLa cells treated with 15 μM nano-curcumin were destructed through the apoptosis process. Free curcumin with a higher concentration (50 μM) could induce apoptosis in 25.9% of cells (Figure 2).
Flow cytometric diagram obtained from annexin/PI test to evaluate cellular apoptosis: A, Apoptosis assay results for cancerous HeLa cells after treatment with 15 μm of nano-curcumin and 50 μm of curcumin. Apoptosis assay results for fibroblast cells in the same condition. B, 46.5% of HeLa cells treated with 15 μM nano-curcumin were destroyed through apoptosis. Free curcumin at a higher concentration (50 μM) could induce apoptosis in 25.9% of cells. The apoptosis test for fibroblast cells showed that nano-curcumin (15 μM) and curcumin (50 μM) had no significant effect on cell apoptosis of normal cells. (Significant at * P < 0.05, ** P < 0.01, *** P < 0.001).
The apoptosis test for fibroblast cells indicated that nano-curcumin (15 μM) and curcumin (50 μM) had no significant effect on the cellular apoptosis of the normal cells (Figure 2).
4.3. Nanocurcumin Regulation Effect on E6, E7, P53, and Rb mRNA Expression
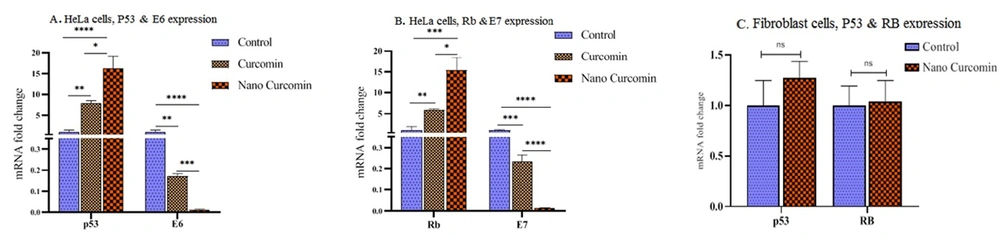
The expression of E6, E7, P53, and Rb genes was measured in HeLa cells. Due to the absence of E6 and E7 oncogenes in fibroblasts, expression of P53 and Rb was measured in these cells under curcumin and nano-curcumin treatment. The results showed that E6 and E7 expressions decreased after 48 hours of curcumin and nano-curcumin treatment in HeLa cells, whereas P53 and Rb expressions were enhanced after this time at mRNA level with P < 0.05 (Figure 3A and B). These findings represented the positive regulatory effect of nano-curcumin on cancer cell apoptosis. The expression of P53 and Rb genes was not significantly altered in nano-curcumin-treated fibroblasts (Figure 3C). Therefore, nano-curcumin did not lead normal fibroblast cells to the apoptosis process.
By real-time RT-PCR, the expression analysis of E6, E7, P53, and Rb genes in cancerous HeLa cells and fibroblast cells. A and B, A significant decrease in the expression of E6 and E7 genes and increase in the expression of P53 and Rb genes were observed in HeLa cells; C, No significant variation was occurred by nano-curcumin treatment of Rb and P53 genes expression in normal fibroblast cells (significant at * P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
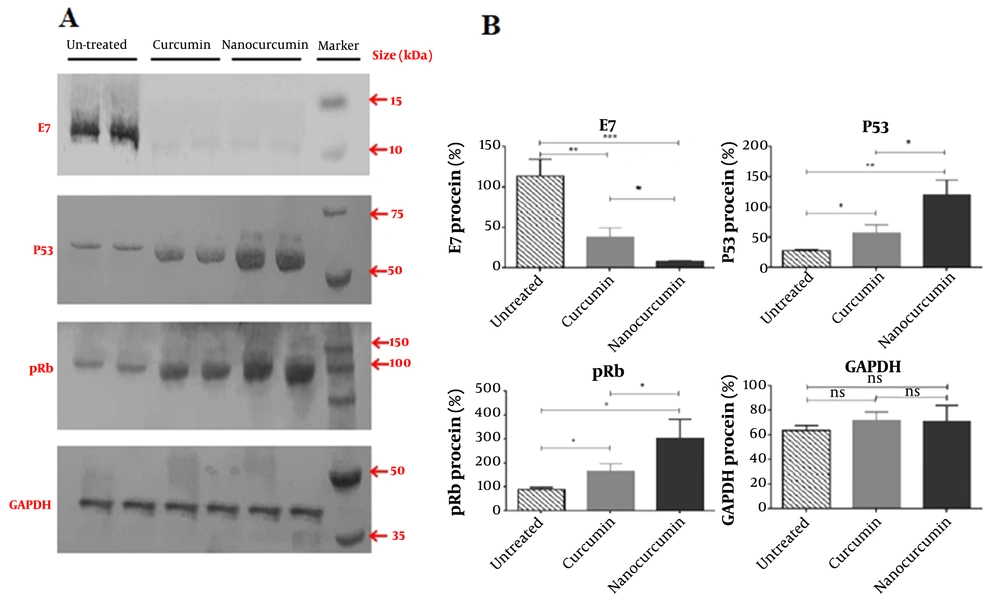
4.4. Nanocurcumin Regulation Effect on E7, P53, and Rb Proteins
The expression of E7, P53, and Rb proteins was analyzed via western blot assay. The results showed that the E7 protein level was reduced in nano-curcumin-treated HeLa cancer cells after 48 hours (P < 0.05). The Rb and P53 proteins increased in these cells (P < 0.05) (Figure 4).
Western blotting results of E7, P53, Rb, and GAPDH protein (as control) in nano-curcumin and curcumin-treated HeLa cells. A, Nanocurcumin treatment can decrease E7 protein expression and increase P53 and Rb proteins in cancerous HeLa cells more than curcumin significantly; B, Data has quantified by ImageJ software, and the significance of protein alterations was determined (significant at * P < 0.05, ** P < 0.01, *** P < 0.001).
5. Discussion
Curcumin is also well-known as an anti-inflammatory and chemopreventative agent (24). Studies in the last three decades about curcumin indicate poor bioavailability, hydrophobicity, poor cellular uptake, and rapid metabolism of this component (25). Various experiments have been performed to solve this problem, many of which have performed nano-carriers (26). Nanotechnology employs curative agents at the nanoscale level to develop nanomedicines. Nanostructures could be utilized to deliver drugs to target tissues, specifically with controlled cell release. Also, in treating cancers, they can increase drug absorption inside tumor cells and improve biodistribution and accumulation in tumor sites (27, 28).
Previous studies have examined the anticancer properties of nano-curcumin made by curcumin and OA400 nano-carrier in various cancers (6, 10, 29). This curcumin nanoformulation can affect different cellular pathways, including differentiation and cell proliferation (2, 30). In vivo studies indicated that treating BALB/c tumor-bearing mice with nano-curcumin could more effectively and efficiently reduce tumor size than curcumin (10). Due to the high prevalence of cervical cancer, drug resistance, and systemic toxicity of conventional chemotherapy and radiation therapy, it is necessary to identify safe, effective, and chemopreventive anticancer natural compounds. Therefore, in the present study, we investigated the potential therapeutic effects of the new nano-curcumin on cervical cancer. In this regard, we evaluated the expression of E6 and E7 human papillomavirus oncogenes and P53 and Rb factors in the HeLa cell line.
Moreover, cell viability and apoptosis rate were assessed in normal and cancer cell lines. Our findings indicated that nano-curcumin could increase cellular apoptosis and reduce cell viability in human cervical cancer HeLa cells. Moreover, the significant overexpression of P53 and Rb genes and under-expression of E6 and E7 was found in HeLa cells treated with nano-curcumin in lower concentrations than curcumin; however, in fibroblast cells that treated by nanocurcumin, the expression of P53 and Rb genes did not altered significantly.
Based on a comparison between the effects of curcumin and nano-curcumin on HeLa cell viability and apoptosis, it was found that nano-curcumin is much more effective than curcumin. Nanocurcumin progressed the apoptosis process in HeLa cancer cells; however, it had no significant effect on normal fibroblast cells. This finding has been demonstrated in previous studies for other cancer cells (6, 8, 31, 32) and may happen because nano-curcumin has higher cellular uptake and biostability than curcumin. Furthermore, HeLa and fibroblasts were treated separately with an OA400 nano-carrier, and it was observed that the carrier had no significant effect on cell apoptosis or cellular viability. The non-toxic function of the nano-carrier proves its safety. This component at very high concentrations (160 μM) reduced fibroblast cell viability by approximately 50%. This amount was not a functional concentration. Therefore, it can be concluded that nano-curcumin is safe for normal cells at the studied concentration. Several reasons explain why curcumin does not affect normal cells. It has been shown that the reduction of intracellular glutathione in cancer cells by buthionine sulfoximine increases ROS levels and makes these cells more sensitive to curcumin (23). Curcumin also has target molecules that are more common in cancer cells (33). In previous studies, the capability of nano-curcumin to induce cancerous and undifferentiated cell apoptosis was detected in a time and dose-dependent manner, while on normal cells, no significant effects were observed (6, 31, 34). HeLa cells are derived from a woman’s aggressive glandular cervical cancer and are used in many studies to evaluate cervical cancer treatment methods (35). E6 and E7 oncogenes in the HeLa cell line are derived from the HPV genome. The integration of HPV type 16 or 18 DNA into the HeLa cells genome has been proven in previous studies. Human papillomavirus type 18 transcripts in the 8q24 chromosome region of the HeLa cell line (36).
E6 and E7 play a central role in the pathogenesis of HPV. These genes encode proteins that bind to P53 and Rb tumor suppressors, respectively, and suppress them. This phenomenon disrupts cell cycle regulation. The expression of these two viral oncogenes is essential for cervical cancer tumorigenesis (37). In this study, the expression of E6 and E7 viral genes was significantly reduced in HeLa cells during treatment with nano-curcumin compared to curcumin. As expected, following the down-regulation of E6 and E7 genes, the expression level of P53 and Rb tumor suppressors increased both at the RNA and protein levels. Thus, nano-curcumin provides a condition for the progression of apoptosis in HeLa cancer cells and can be considered a safe complementary drug for cervical cancer. In line with our findings, another type of nano-curcumin (poly (lactic-co-glycolic acid) based curcumin nanoparticle formulation) has been applied to apoptosis induction in cervical cancer. Results indicated that this type of nano-curcumin effectively inhibits the growth of cervical cancer cell lines in 20 μM and 25 μM, abrogates expression of E6 and E7 oncogenes, and leads them to apoptosis (21). In another study, curcumin concentrations above 40 μM induced apoptosis in HeLa cancer cells (38). However, inconsistent with the present study that revealed the functional effect of nano-curcumin in a low concentration (15 μM), other studies activated apoptosis with higher concentrations. This observation may confirm that the nano-curcumin was made using the OA400 nano-carrier works more effectively and has more cellular uptake than curcumin.
5.1. Conclusions
According to the results of the present study, nanoformulation of curcumin containing OA400 nano-carrier can suppress HPV oncogenes, restore P53 and Rb proteins and induce apoptosis of human cervical cancer HeLa cells more than curcumin. Therefore, this nanostructure of curcumin has significant potential to be used as a complement drug for cervical cancer treatment. The improvement of the anti-proliferative function of curcumin in different cancer cells can occur by using nano-types. Nanocurcumin, as a safe and herbal drug, may have the potential to be used as a supplement along with common cervical cancer treatment strategies such as chemotherapy drugs.