1. Background
Contact dermatitis is an inflammatory skin disease caused by skin exposure to an irritant (1, 2). Based on the pathophysiological mechanisms, this disease has two main types: Irritant contact dermatitis (ICD) and allergic contact dermatitis (ACD) (3). The mechanisms of ICD and ACD differ, at least in the starting stage of inflammation. In ACD, after contact with the irritant, a sensitivity phase is first created, and from the second contact onwards, acquired immunity is activated. Unlike ACD, after the first contact with the stimulating substance in ICD, innate immunity is activated and causes inflammation, although it eventually activates acquired immunity after repeated encounters (4). The following steps that lead to the development of eczema lesions are very similar in ACD and ICD in that both have a similar production of cytokines, chemokines, cell infiltration, apoptosis, and necrosis. This causes ACD and ICD lesions to be clinically similar and confused with each other (4).
Due to various environmental irritants in work environments, ICD is one of the most common occupational diseases today. As a result, ICD is one of the most critical problems from a health and economic point of view (5). On the other hand, there are no effective treatments for ICD so far. As current treatments have only tried to improve the symptoms of the disease (6), considerable research has been done to reveal and elucidate the complex pathophysiology of ICD and develop potential drugs.
During research to discover new drugs, it is always necessary to use animals to check the candidate drug’s effectiveness and safety. Human ICDs can be accurately mimicked using mouse models. These mouse models of skin sensitivity have provided a powerful tool to study the causes and mechanism of the disease and carry out therapeutic interventions (5).
The ICD model can be created in several ways: Using mice that spontaneously develop ICD-like skin lesions, creating genetically modified mice that overexpress or have defects in the expression of molecules involved in the disease, and using chemicals to create skin lesions similar to ICD lesions (5). Creating a genetically modified mouse model is a costly process. On the other hand, the genetically modified mouse model may not always show the desired phenotype, and the studies conducted with these models may not be easily generalized to humans. Hence, not all researchers can prepare this type of mouse model for study (7).
The other important point is that we should use the minimum number of animals in such experiments. We should also use protocols that cause minimal animal pain and stress. Thus, we must use standardized methods with acceptable results that impose the least pain and do not bother the animals. On the other hand, the protocol used should be simple, reliable, repeatable, and as affordable as possible for the researcher. Therefore, developing such methods is one of the most important research priorities. A better understanding of the mouse model of ICD will enable researchers to focus their studies on the pathogenesis of human ICD and develop new therapeutic targets by precisely identifying the disease mechanism.
2. Objectives
In this study, we intend to create a mouse model of ICD with the least possible difficulty and stress in mice and discuss the consequences of mechanical skin damage and skin barrier dysfunction in skin inflammation.
3. Method
3.1. Animals
The animals were three-week-old female BALB/c mice purchased from the Pasteur Institute of Iran. They were maintained under conventional and controlled conditions in the holding facility on a regular 12: 12 h light/dark cycle (lights from 7:00 AM to 7:00 PM), a constant temperature of 21 ± 1°C, and 55 ± 5% humidity. Mice were housed in transparent plexiglas cages and were allowed to acclimate to the housing conditions for one week before the start of experimental procedures. Mice had ad libitum access to standard food and water. Animal discomfort was minimized during all procedures.
All experiments were performed according to the guidelines for the care and use of laboratory animals. The Ethics Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran, approved the standard protocols. In this study, we tried to use the minimum number of animals and the minimum amounts of irritants.
3.2. Sensitization of Mice with Dinitrochlorobenzene
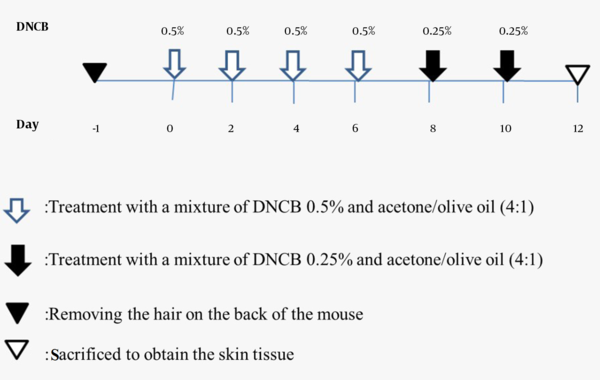
Fifteen mice were used in this study. They were divided into three groups (n = 5 per group): (1) healthy control group, (2) healthy control group treated with acetone/olive oil, and (3) group treated with a mixture of dinitrochlorobenzene (DNCB) and acetone/olive oil. Before starting the experiment, the back hair of the animals with a diameter of 2 cm was removed entirely with an electric shaver. Then, on days 0, 2, 4, and 6, a 50 μL mixture of 0.5% DNCB and acetone/olive oil (4: 1) was applied to the shaved zone. On days 8 and 10, a 25 μL mixture of 0.25% DNCB and acetone/olive oil (4: 1) was applied to the back of the mouse. After each treatment, we kept the animal outside the cage for a few minutes to dry the solution. The experimental schedule is presented in Figure 1.
3.3. Histological Analysis
At the end of the study, the animals were humanly euthanized under anesthesia, and the dorsal skin tissues were separated on the 10th day. Skin tissues were placed in 10% neutral formalin (Merk, Germany) and then fixed in paraffin (Merk, Germany). Sections with a thickness of 6 μm were prepared and stained with Hematoxylin and Eosin (Merk, Germany).
4. Results
4.1. A Mixture of Dinitrochlorobenzene 0.5% with Acetone/Olive Oil Causes Clinical Features Like Irritant Contact Dermatitis
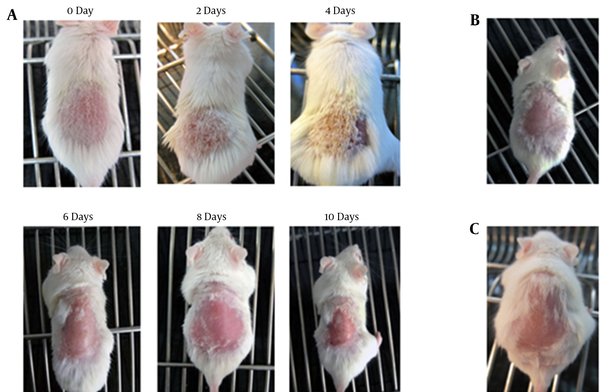
As a result of applying DNCB 0.5% with acetone/olive oil, mice developed contact dermatitis over 10 days. The following pictures show the skin changes in mice clinically from the beginning to the end of the treatment period (Figure 2).
Clinical characteristics of mice in different groups. A, Clinical features of irritant contact dermatitis (ICD) symptoms induced by dinitrochlorobenzene (DNCB) and acetone/olive oil mixture in mouse model on days 0, 2, 4, 6, 8, and 10; B, Clinical characteristics of untreated healthy control mice; C, Clinical features induced by acetone/olive oil mixture in mice on day 10.
4.2. The Mixture of Dinitrochlorobenzene 0.5% with Acetone/Olive Oil Causes Histopathological Changes Similar to Irritant Contact Dermatitis
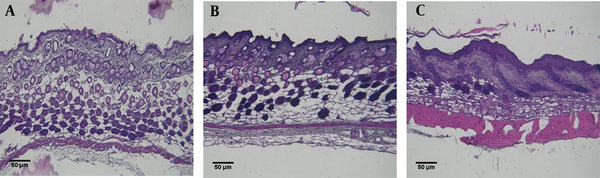
Pathological studies confirmed the occurrence of irritant contact dermatitis in mice. As shown in Figure 3, tissue hyperemia, tissue thickening, and leukocyte infiltration are observed in the irritant contact dermatitis group compared to the healthy control group (Figure 3).
Histopathological results of mouse back skin tissues in A, Untreated healthy control mice; B, Mice treated with a mixture of acetone/olive oil; C, Irritant contact dermatitis (ICD) mice treated with a mixture of dinitrochlorobenzene (DNCB) and acetone/olive oil. Dorsal skin sections were stained with H&E (Scale bars = 50 μm).
5. Discussion
Irritant contact dermatitis is an inflammatory skin disease, also known as “primary contact hypersensitivity (CHS)”, which develops following skin exposure to potent haptens such as urushiol, primin, 2,4-dinitrofluorobenzene (DNFB), and DNCB. After the skin is exposed to a hapten, pro-inflammatory mediators will be released, attracting inflammatory cells to the damaged tissue (8).
The study of mouse models of ICD has clarified the pathogenesis of skin inflammation. These studies will clarify the complex mechanism of the disease and determine the role of each of the mediators and cells in response to the irritant and disease development (9). Therefore, a better understanding of the mechanisms of these models will eventually lead to the creation of a broader range of therapeutic interventions for this prevalent and potentially disabling disease.
As known, DNCB and other chemicals with a similar structure, like DNFB and picryl chloride, act as immunogens and cause irritancy in the mouse’s skin. Various chemical agents can be used as an irritant to induce ICD in mice. One of the most commonly used chemical irritants is DNFB (10-12). These immunogens are recognized by local APCs, like Langerhans cells and macrophages. Then, they are processed and presented to T cells. Repeated exposure to that immunogen can cause dermatitis (13-15). One of the critical factors for all chemicals to cause ICD and ACD is their concentration (16, 17). For example, DNFB is an irritant at a concentration of 0.05%, but geraniol is an irritant at 50% (18). In the study of Zhang et al. (2), a 100 μL mixture of 0.5% DNCB and acetone/olive oil (3: 1) was applied to the shaved area for one and two days. In another study, to create a mouse model of ICD, a 20 μL mixture of 0.5% DNCB with olive oil/acetone (4: 1) was applied to the left ear of mice (8). Whereas our study used a 50 μL mixture of 0.5% DNCB and acetone/olive oil (4: 1) on days zero, two, four, and six, and then a 25 μL mixture of 0.25% DNCB and acetone/olive oil (4: 1) was applied on the back skin. Finally, we induced irritant contact dermatitis models after the 10th day of DNCB application. Nevertheless, in the study of Zhang et al. (2), atopic dermatitis models were induced after 28 days of DNCB application. Furthermore, DNCB was used to induce atopic dermatitis at different dilutions and for longer periods (19, 20).
Gaspari et al. (21) showed that a mixture of acetone/olive oil and DNFB causes significant damage to the skin. Also, acetone/olive oil alone can cause skin damage. However, our study did not see any skin inflammation in macroscopic and pathologic evaluations of the healthy control group treated with acetone/olive oil. In addition, using acetone/olive oil as a solvent for DNCB worked very well. However, a researcher should be aware of skin erosions when using acetone/olive oil, especially with DNFB.
In general, the model created with DNFB is a powerful tool for studying ICD due to its reproducibility in different mouse species. On the other hand, DNCB does not pose a high threat to the general health status of mice. Therefore, it can be advantageous to prepare a laboratory model of skin diseases.
5.1. Conclusions
The protocols used to create mouse models should impose minimal distress and pain on the animal. In this study, we introduced a protocol that, while simple, could effectively create an ICD mouse model with much less stress and pain than conventional methods for mice. This approach effectively increased the scratching behavior of mice and caused ICD-like skin lesions macroscopically and histopathologically. Also, this ICD mouse model is simple, reliable, and reproducible. On the other hand, the created mouse model is economical and feasible for all researchers. We hope this study will help other laboratories to provide new perspectives on drug development for ICDs and adjust their models so that animals experience the least possible pain and stress.