1. Background
Sarcoidosis is one of the chronic inflammatory diseases with unknown etiology and involvement of multiple organs. The characteristic of sarcoidosis is the granulomatous inflammation with a local development of activated CD4 + Th1 cells and macrophages in the affected organs (1). Comprehensive symptoms include weight loss, muscle pain, night sweats, reduced exercise capacity, fever, cough, and dyspnea, which eventually lead to poor quality of life (QoL) in patients (2). A prevalent symptom in these patients is fatigue, which is related with poor sleep quality and excessive daytime sleepiness (EDS) (3).
Sarcoidosis and sleep-disordered breathing (SDB) can cause fatigue symptoms. Generally, 60% of patients with obstructive sleep apnea (OSA) suffer from fatigue. Moreover, the prevalence of fatigue in sarcoidosis patients is equivalent (50 - 70%) to patients with OSA (4). In addition, fatigue in sarcoidosis can be caused by inflammatory mediators generated during granulomatous inflammation, as well as unfavorable responses to corticosteroid therapy or its discontinuation (5). Interrupted dreams and hypoxemia are the causes of fatigue in SDB (6). On the other hand, sarcoidosis causes sleep disorders (6, 7). Studies showed that OSA in sarcoidosis patients is more common than in healthy people (8). Etiology of sleep disorders in sarcoidosis patients include decreased sleep quality based on the respiratory symptoms of pulmonary sarcoidosis (2), sleep disturbance arising from granulomatous inflammation of the upper airway (9), weight gain induced by corticosteroids consumption (10), and finally, infiltration of hypothalamus through neurosarcoidosis can occur. The hypothalamus elaborates in regulating sleep and circadian function. (11).
Excessive daytime sleepiness is another symptom of sleep disorder which leads to dysfunction in typical life situations and also decreases the quality of life in sarcoidosis patients (12).
The pineal gland primarily releases melatonin (sleep hormone) at night and associated with control of the sleep-wake cycle. It is essential in regulating human circadian rhythms and may have hypnotic activity in humans (13). In order to treat sleep disorders, melatonin administration by binding to particular receptors, can mimic the function of endogenous melatonin and trigger downstream (14). Immunomodulatory and anti-oxidant features of melatonin are already established (15). Exogenous melatonin’s efficacy in improving sleep quality in several medical conditions, such as Chronic Obstructive Pulmonary Disease (COPD), Chronic Pulmonary Sleep Apnea (CPSA), and asthma has been evaluated (16-18). Several studies have used melatonin as an agent for the treatment of sleep disorders such as primary insomnia, chronic sleep-onset insomnia and delayed sleep phase syndrome, however, the beneficial effects remain controversial (19).
2. Objectives
This study aimed to evaluate if a 3-month melatonin treatment could affect subjective sleep parameters in pulmonary sarcoidosis patients with sleep problems.
3. Methods
3.1. Randomized Clinical Trial Development and Enrollment
A randomized, single-blinded clinical trial was conducted in the Masih Daneshvari Hospital, a university hospital in Tehran, Iran, on patients with pulmonary sarcoidosis referred to the sarcoidosis clinic for one year.
The study was approved by the Ethics Committee of the Shahid Beheshti University of Medical Sciences, Tehran, Iran (Ethics code: IR.SBMU.PHARMACY.REC.1399.339) with registry code of IRCT20151227025726N25 in the Iranian Registry of Clinical Trials (IRCT).
The sample size was calculated based on 80% of power, four standardized differences, and α (type I error) of 0.05. Therefore, the utmost number was selected for the sample size based on the Pittsburgh Sleep Quality Index (PSQI) score. Informed written consent was obtained from all patients before random allocation of the study groups.
All patients (age > 18 years) with pulmonary sarcoidosis and sleep-related disorders (PSQI > 5) (20) were enrolled in the study.
Exclusion criteria were breastfeeding, pregnancy, neurological diseases (epilepsy, stroke, previously neurologic manipulation and, tumor), psychiatric diseases (depression, anxiety), using any hypnotic or sedative drugs (antihistamine drugs, benzodiazepines), use of stimulant medications, history of allergy to melatonin or formulation components, having parasomnia, shift work sleep disorder, and fluvoxamine consumption.
A block randomization method (twenty-five blocks, including four patients in each block) was used to allocate patients to the melatonin and control groups. Two patients were assigned to the melatonin group in each block and two to the control group. As a single-blind study, the investigator was blind; however, outcome assessors and data analysts were also blind to the study groups until the end of the study and interpretation of the findings.
3.2. Intervention
The patients in the melatonin group received 3 mg melatonin tablet (batch No. 0006VMX0327, Razak Pharmaceutical Company, Tehran, Iran), q.d., one hour before sleep for three months, and were given sleep hygiene guidance based on the Center for Disease Control and Prevention (CDC) recommendations (21). The control group only received sleep hygiene advice. All patients were allowed to leave the study at any time during the study and received any sedative-hypnotic drugs. The patients’ demographic characteristics, disease severity, drug history, and BMI were also documented. During the 90-day study period patients were also trained to keep a diary of their sleep and wake time.
3.3. Outcome Measures
As primary outcomes, sleep quality, daytime sleepiness, fatigue status, and quality of life were assessed.
To evaluate sleep quality, the General Sleep Disturbance Scale (GSDS) and PSQI were measured (20, 22).
The General Sleep Disturbance Scale is a 21-item questionnaire that practices the entirety of points (0 - 7 points per question), specified as the “sleep disturbance score,” to define four groups: no disturbance (0 - 21 points), rare disturbance (22 - 42 points), occasional disturbance (43 - 63), and frequent disturbance (64+ points). GSDS is confirmed in healthy individuals and patients with chronic diseases (patients with HIV, Parkinson’s disease and cancer) (23).
The Pittsburgh Sleep Quality Index is a scale that contain seven components, each one dealing with a significant aspect of sleep: (1) sleep latency; (2) subjective sleep quality; (3) sleep efficiency; (4) sleep duration; (5) sleep disturbance; (6) daytime dysfunction; and (7) use of sleep medication. These modules are weighed equally on a 0 - 3 scale, with a global score of 0 - 21. A global PSQI score above five was found to have a sensitivity of 89.6% and specificity of 86.5% to differentiate well from poor sleepers (20).
The Epworth Sleepiness Scale (ESS) was practical to evaluate daytime sleepiness. ESS is a questionnaire that measures a subject’s expectation of dozing in eight hypothetical states. Dozing probability ratings range from zero (none) to three (high probability). A total score of 11 or more is considered to indicate excessive daytime sleepiness (24).
Two instruments, the Fatigue Assessment Scale (FAS) and Patient-Reported Outcomes Measurement Information System (PROMIS), were measured for fatigue assessment. The FAS is a 10-item patient-reported outcome questionnaire that has verified validity in the general population and many of sarcoidosis patients. The 10-item PROMIS questionnaire is a valid and reliable ration to assess fatigue in sarcoidosis patients. Each item is valued on a Likert scale of “never” (1 point) to “always” (5 points) scale. Both tools cut across subjects into either experiencing or not experiencing fatigue (FAS: ≥ 21 points, PROMIS: ≥ 18 points (23)).
To assess the quality of life of the patients, 12-item Short Form Survey (SF-12) questionnaire was used.
Twelve-item Short Form Survey is a broadly used tool that assessed quality of life and scored into the Mental Component Summary (MCS) score and the Physical Component Summary (PCS) score. The 12-item form combines several different provisions, including dichotomized “yes” or “no” answers and scales that rate the frequency and severity of emotional responses and physical abilities (25). Higher scores indicate improved quality of life in each category. Twelve-item Short Form Survey is validated across several adult cohorts in the United States and internationally, with a score of 50 considered a normative value for the US individuals (26).
The above outcomes were measured and recorded on day 1 (baseline) and three months after treatment. Besides, patients were followed by phone calls once a week to evaluate their adherence, ease of use, and possible adverse reactions to melatonin.
3.4. Statistical Analysis
The statistical investigates were accomplished using SPSS software for Windows (version 23.0; SPSS Inc., Chicago, IL, USA). Categorical and nominal variables were stated as frequency (%) and were compared using the chi-square test. Continuous variables were tested for normal distribution by Kolmogorov-Smirnov test. Data were stated as means ± standard deviations or median [interquartile range], depending on the variable’s normal or non-normal distribution. For data with the normal and non-normal distributions, appropriate parametric (sample t-test) and non-parametric (Mann-Whitney test) tests were used, respectively. P-values < 0.05 were considered as significance level.
4. Results
4.1. Demographics and Clinical Characteristics
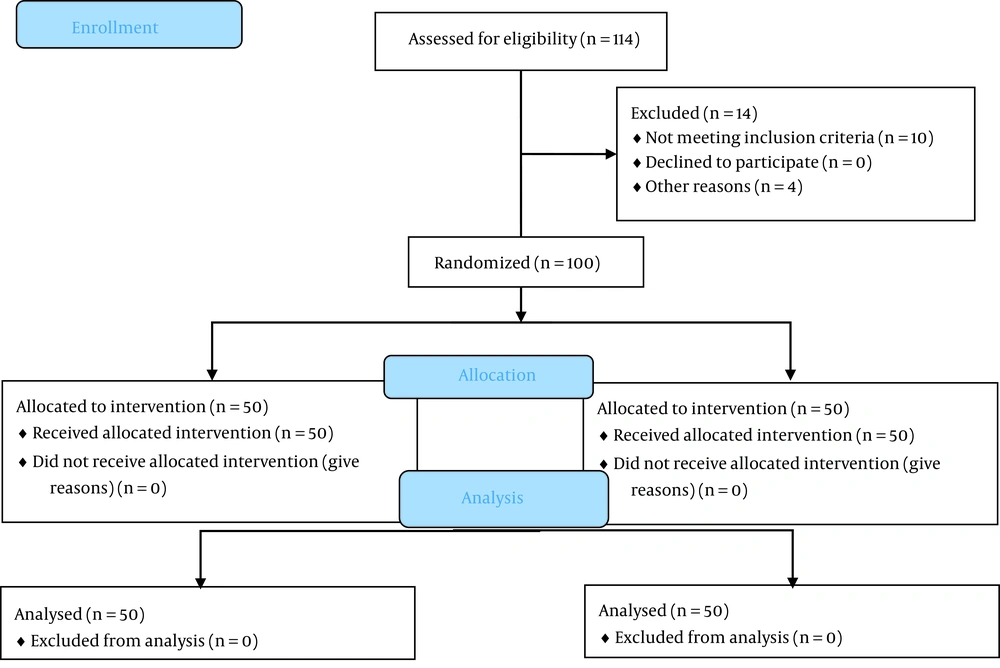
One hundred and fourteen patients were eligible to enroll in the study. Fourteen patients were excluded due to not meeting inclusion criteria and not signing the written informed consent. In general, 100 patients (equally allocated in the study groups) finished the study (Figure 1). The demographics and clinical characteristics of the patients are shown in Table 1. Immunosuppressive treatments were used by both groups, with prednisolone being the most common medicine.
| Characteristics | Melatonin | Control | P-Value |
|---|---|---|---|
| Gender | 1 | ||
| Male | 15 (30) | 15 (30) | |
| Female | 35 (70) | 35 (70) | |
| Age (y) | 49.9 ± 9.70 | 51.4 ± 9.30 | 0.40 |
| BMI (kg/m2) | 23.87 ± 4.20 | 24.10 ± 4.00 | 0.80 |
| Scadding stage | 0.90 | ||
| 0 | 1 | 1 | |
| I | 28 | 27 | |
| II | 18 | 21 | |
| III | 3 | 1 | |
| IV | 0 | 0 | |
| Medications | |||
| MTX | 28 | 25 | - |
| Prednisolone | 50 | 50 | - |
| Hydroxychloroquine | 16 | 20 | - |
| Mycophenolate mofetil | 1 | 0 | - |
| Past medical history | |||
| Diabetes | 1 | 2 | - |
| Hypertension | 0 | 3 |
Abbreviations: BMI, body mass index; MTX, methotrexate; Scadding stage 0, No chest abnormality; I, hilar lymphadenopathy; II, hilar lymphadenopathy and parenchymal abnormality; III, parenchymal abnormality without hilar lymphadenopathy; IV, fibrosis with volume loss.
a Values are expressed as No. (%) or mean ± standard deviation.
4.2. Sleep Quality Assessment
One of our main outcomes was to determine sleep quality based on GSDS and PSQI scales. The baseline values of the melatonin and control groups are presented in Table 2.
| Score | Melatonin | Control | P-Value |
|---|---|---|---|
| FAS score | 31.94 ± 9.53 | 28.46 ± 8.10 | 0.05 |
| Global Physical Health Raw Score | 12.52 ± 2.54 | 12.22 ± 2.40 | 0.54 |
| Global Mental Health Raw Score | 11.34 ± 3.32 | 11.54 ± 2.83 | 0.74 |
| PCS-12 score | 37.8 ± 7.92 | 38.89 ± 7.86 | 0.48 |
| MCS-12 score | 39.43 ± 10.86 | 40.67 ± 11 | 0.57 |
| PSQI score | 11.88 ± 3.99 | 10.54 ± 4.07 | 1.00 |
| ESS score | 7.34 ± 5.42 | 5.78 ± 4.76 | 0.13 |
| GSDS score | 48.10 ± 20.59 | 35.34 ± 21.23 | 0.003 |
Abbreviations: FAS, Fatigue Assessment Scale; PCS-12, Physical Score; MCS-12, Mental score; PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Scale; GSDS, General Sleep Disturbance Scale.
a Values are expressed as mean ± standard deviation.
Among 21 items on GSDS, those components related to difficulty getting to sleep, awakening during sleep, and waking up too early from sleep were scored higher in the control group than in the melatonin group. As shown in Table 2, at baseline, the melatonin group (48.10 ± 20.59) was experiencing worse occasional sleep disturbance compared to the control group (35.34 ± 21.23) (P = 0.003). However, after three months of melatonin therapy, group analysis revealed that the GSDS score declined significantly (P < 0.001) in the melatonin group (-16.52 ± 13.6) compared to that of the control group (-2.14 ± 10.77) (Table 3).
| Variations | Melatonin | Control | P-Value |
|---|---|---|---|
| FAS score change | -8.28 ± 9.73 | -1.86 ± 3.79 | < 0.001 |
| P-value (within-groups) | < 0.001 | 0.002 | |
| Global Physical Health Raw Score change | 0.84 ± 1.82 | -0.06 ± 1.33 | 0.006 |
| P-value (within-groups) | 0 .002 | 0.75 | |
| Global Mental Health Raw Score change | 0.86 ± 2.53 | -0.08 ± 1.20 | 0.02 |
| P-value (within-groups) | 0.02 | 0.64 | |
| PCS-12 score | 3.38 ± 4.61 | 0.55 ± 7.25 | 0.02 |
| P-value (within-groups) | < 0.001 | 0.59 | |
| MCS-12 score | 2.99 ± 70 | 0.25 ± 8.16 | 0.07 |
| P-value(within-groups) | 0.003 | 0.83 | |
| PSQI score | -3.36 ± 4.27 | -0.20 ± 3.79 | < 0.001 |
| P-value (within-groups) | < 0.001 | 0.71 | |
| ESS score | -3.02 ± 4.07 | -0.52 ± 3.9 | 0.002 |
| P-value (within-groups) | < 0.001 | 0 .35 | |
| GSDS score | -16.52 ± 13.6 | -2.14 ± 10.77 | < 0.001 |
| P-value (within-groups) | < 0.001 | 0.17 |
Abbreviations: FAS, Fatigue Assessment Scale; PCS-12, Physical Score; MCS-12, Mental score; PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Scale; GSDS, General Sleep Disturbance Scale.
a Values are expressed as mean ± standard deviation.
At the start of the study, the mean ± SD PSQI scores for the melatonin and control groups were 11.88 ± 3.99 and 10.54 ± 4.07, respectively (P = 1.00). All subjects (100%) had PSQI scores > 5, indicating poor sleep quality. Global PSQI score was significantly decreased in melatonin (3.36 ± 4.27) compared to control group (0.2 ± 3.79) (P < 0.001) (Table 3).
4.3. Daytime Sleepiness Assessment
Baseline ESS scores for the melatonin and the control groups were 7.34 ± 5.42 and 5.78 ± 4.76, respectively. There was a statistically significant reduction (P = 0.002) in ESS score in the melatonin group (-3.02 ± 4.07) compared to that of the control group (-0.52 ± 3.9) after three months of therapy. Between-groups (P = 0.002) and within-groups comparisons (P < 0.001) for ESS score were significant (Table 3).
4.4. Assessment of Fatigue and Quality of Life
The fatigue assessment was conducted with the measurement of FAS and PROMIS scores. Compared to control, significant differences were observed in FAS scores after three months of therapy (P < 0.001). FAS score was decreased by 8.28 ± 9.73 in melatonin and 1.86 ± 3.79 in the control group. As well significant differences were found in the PROMIS questionnaire. PROMIS questionnaire is divided into global physical health and global mental health raw scores. After the intervention¸ global physical health and global mental health raw scores were improved (P = 0.006, and P = 0.02, respectively).
Evaluating the quality of life with SF-12 showed that mean of PCS (41.18 ± 8.89) and MCS (42.43 ± 10.13) were increased by 3.38 ± 4.61 and 2.99 ± 70 in the melatonin group compared to the control group (P < 0.001, and P = 0.003, respectively).
Statistically significant differences were found between melatonin and control groups in PCS-12 scores after three months of therapy (P = 0.022). Although the MCS-12 score was increased at the termination of the study, this increase was not significant (P = 0.074).
4.5. Adverse Effects
Thirteen patients (out of 100 patients) reported mild adverse effects, including mild headache (11 subjects in the melatonin group) and mouth dryness (1 subject in the melatonin group and one in the placebo group) which were not clinically important.
5. Discussion
Melatonin is available over the counter or by prescription in several countries without reporting significant adverse reactions (18). There is no definite information on the exact pathophysiology of fatigue, poor sleep quality, and excessive daytime sleepiness in patients with sarcoidosis. However, some research showed that decreased sleep quality is multifactorial; the respiratory symptoms of pulmonary sarcoidosis, granulomatous inflammation of the upper airway, weight gain induced by corticosteroids consumption, and finally, neurosarcoidosis caused poor sleep quality and excessive daytime sleepiness. Melatonin with immunomodulatory and anti-oxidant features can improve sarcoidosis symptoms and consequently reduce sarcoidosis-related fatigue. Furthermore, in some respiratory studies such as, COPD, chronic pulmonary sleep apnea, and asthma, the use of melatonin can improve sleep parameters such as sleep quality. Based on these evidences, melatonin could affect subjective sleep parameters in pulmonary sarcoidosis patients with sleep problems (9-11, 15-18). The rate of poor sleep quality (global PSQI score > 5) was 67% in sarcoidosis patients (27), and this is a common problem. Accordingly, it is critical to use newer agents to help these people with night’s sleep. We found that supplemental (exogenous) melatonin may be associated with improving quantity and quality of sleep in patients with pulmonary sarcoidosis. Nunes et al. (18) surveyed the consequence of melatonin administration on subjective sleep quality, measured by PSQI, in patients with chronic obstructive pulmonary disease. They found that melatonin could improve global PSQI, particularly sleep latency and duration components. Similarly, our study showed that melatonin could significantly decrease PSQI and improve sleep quality and latency in sarcoidosis patients. The evaluation of melatonin in the meta-analysis showed that it might be effective for improving sleep onset latency and could be combined with cognitive-behavioral therapy (CBT) to get a better response. The evidence suggests that melatonin can enhance sleep quality and daytime activity, restore the sleep/wake cycle and sustain the physiological sleep structure in insomnia patients (28). The study by Selvanathan et al. showed that CBT-I could improve sleep quality without improving anxiety symptoms and fatigue in chronic pain patients (29). In the systematic review of Sumsuzzman et al., chronic nocturnal melatonin treatment improved cognition without any unfavorable effects (30). Daytime cognitive performance was not impaired by melatonin and consistently improved cognition with melatonin treatment compared to placebo (30).
A study by Jafari-Koulaee and Bagheri-Nesami suggests that insomnia and sleep quality among cancer patients can improve with the administration of melatonin (31). However, its duration and dose of use be subject to patients’ circumstances and features. Based on the evidence acquired from the present study, it may be specified that better results can be accomplished in improving patients’ sleep quality by using a higher melatonin dose for a shorter duration of time (20 mg for ten days) during chemotherapy (31).
The dose of melatonin which may improve sleep quality varied between 3 and 20 mg (3 mg in most studies) in studies (32-35). There is no definite evidence on the effective and safe dose of melatonin to improve sleep quality in patients with sarcoidosis. However, some investigators have stated that the typical dose of melatonin to improve sleep quality is between 1 and 5 mg (36). The products also may have different concentration and purity. Therefore, more robust evidence regarding melatonin administration to improve sleep quality is needed, especially in sarcoidosis patients, to afford a precise clinical guide in this field.
Based on the evidence from the present study, the reduction in ESS in the melatonin group compared to the control group (P = 0.002) was highly linked with a drop in daytime drowsiness. According to Hinz et al.’s study (37), EDS is common among sarcoidosis patients (50%), and no specific treatment has been reported to help these individuals in this regard yet. Therefore, based on our results, melatonin could be a new beneficial therapy in most sarcoidosis patients with EDS. Pharmacologic treatment of EDS includes both US Food and Drug Administration (FDA)-approved and off-label medications and may target either the underlying cause or the symptom of excessive sleepiness. To treat EDS in narcolepsy, modafinil, armodafinil, dextroamphetamine, mixed amphetamine/dextroamphetamine, methylphenidate, sodium oxybate, solriamfetol, and pitolisant are FDA-approved (38). Treatment of EDS secondary to medical conditions varies, and small numbers of patients being treated often limit evidence. The AASM practice parameters list modafinil as a treatment option for EDS due to Parkinson’s disease, myotonic dystrophy, and multiple sclerosis. A small study suggests that methylphenidate may be effective for treating EDS secondary to myotonic dystrophy (39).
There is little information on managing tiredness caused by sarcoidosis, and even successful therapies for active sarcoidosis do not eliminate the severe symptoms of concomitant fatigue (40). The FAS score is the only sarcoidosis-specific fatigue scale. It was determined that a four-point change in this scale is a minimal clinically significant change that clinicians and patients would care about (37). Our study observed a significant decrease in FAS score in the melatonin group (8 points reduction) versus only a one-point reduction in the control group. Likewise, van Heukelom et al., in a study on patients with chronic fatigue syndrome, showed that using melatonin could significantly improve fatigue levels (41).
The SF-12 questionnaire was applied to assess the patients’ quality of life in the study groups. MCS score reveals psychological distress, social functioning, and energy levels. In contrast, the PCS score manifests general health perceptions, pain, and physical functioning (25, 26). Our results recommend that greater sleep disturbance levels can be significantly associated with physical functioning impairment (P < 0.001). Furthermore, our study showed that melatonin could significantly improve PCS in sarcoidosis patients. According to Vis et al.’s systematic review, the limited pharmacological treatment for reduced quality of life or fatigue in sarcoidosis patients are available (42). However, the use of melatonin can improve PCS in patients with fibromyalgia (43), delayed sleep phase syndrome (44), and chronic fatigue (45), While administration of melatonin cannot improve PCS in women with osteoporosis (46) and hemodialysis patients (47). In our study, administration of 3 mg melatonin for three months can improve subjective sleep quality. In the study of Russcher et al., administration of 3 mg melatonin for three months in hemodialysis patients can improve subjective sleep problems (47). Also, consuming 3 mg melatonin in COPD patients for 21 consecutive days can improve sleep latency and duration without improving daytime sleepiness (18).
More studies with a longer study period, larger sample size, and different doses of melatonin are recommended to re-confirm our findings and determine if melatonin leads to sustained long-term clinical benefits in patients with sarcoidosis. Another study limitation is lack of evidence about sustained melatonin clinical benefits after discontinuation of medication.
In summary, patients with sarcoidosis experience numerous signs including sleep disturbance and reduced quality of life. Our results showed that melatonin administration with the dose of 3 mg, q.d., for three months could meaningfully improve sleep quality, fatigue, excessive daytime sleepiness and quality of life in patients with pulmonary sarcoidosis.