1. Background
For evading the host immune response, malignant tumors have several mechanisms that lead to T-cell deficiency in the distinction of the tumor, including mutations in genes required for antigen processing, impaired antigen presentation on the tumor surface, and production of immunosuppressive proteins that suppress T-cell activation (1-3). Targeting vital parts of the immune checkpoint pathway leads to substantial outcomes in relevant clinical studies for unleashing reactive T lymphocytes against tumors (4). This approach mainly aims to restitute the T-cell responses to fight tumor cells.
Numerous investigations have been focused on programmed cell death 1 (PD-1) and its ligands (PD-L1 and PD-L2) interaction as one of the main mechanisms tumor cells employed to evade the host immune responses (5). PD-1 is a cell surface receptor expressed on dendritic cells, monocytes, natural killer cells, activated T-cells, and B-cells. It belongs to the CD28/CTLA-4 family, which starts an inhibitory signal in T-cell activation as a costimulatory molecule (6). PD-L1 (B7-H1) and PD-L2 (B7-DC), the cell-surface glycoproteins belonging to the B7 family, have been recognized as the main ligands by PD-1 (7). After T-cell activation by antigen-presenting cells, PD-1 expression is also induced on the T-cell surface. The PD-1/PD-L1 interaction restricts T-cell proliferation and survival, inhibits effector function like cytokine release and cytotoxicity, prompts tumor-specific T cells apoptosis, increases the resistance of tumor cells to cytotoxic T cells attack, and stimulates the Foxp3+ regulatory T cells differentiation from CD4+ T cells (8-10). Interaction of PD-1 on tumor-infiltrating T lymphocytes with the tumor-expressed PD-L1 also results in an impaired function of T-cells, elucidating the failure of the immune system to develop an effective reaction against cancer (11). Suppression of this pathway has displayed enhancements in T cell activation and survival of cancer patients (12). According to these observations, understanding the physiology of the PD-1/PD-L1 interaction and inhibition of this interaction via checkpoint inhibitors (CPIs) have been considered promising strategies for cancer therapy.
Multiple studies have elucidated that raised soluble PD-L1 concentration in the patient's plasma has been associated with the worst prognosis. On the other hand, any post-therapeutic elevation of PD-L1 in the plasma has been associated with an increase in the survival rate for numerous cancers. Local delivery of the PD-1 gene to the tumor microenvironment revealed a rise in the tumor-specific CD8+ T cell immunity and a reduction in tumor growth (13). Moreover, it can diminish the inhibitory effect of soluble PD-L1 and improve immunotherapies based on monoclonal antibodies (14). Thus, elevating the soluble PD-1 level systemically or locally could have a promising perspective for cancer immunotherapy.
To evaluate the local effect of PD-1 protein on the PD-1/PD-L1 interaction in the tumorized-mice model, a recombinant murine PD-1 (mPD-1) was prepared. The outcome revealed a PD-1/PD-L1 pathway suppression by recombinant mPD-1 in terms of tumor size and survival rate in the mice model.
2. Methods
2.1. Animal and Cell
The animal study was conducted according to the ethical standards of the declaration of Helsinki. C57BL/6 female mice (4 - 6 weeks) were obtained from the Pasteur Institute of Iran. The mice were kept for one week before the experiment and fed with free access to water and food under standard conditions. TC-1 cells were cultured in high glucose Dulbecco's Modified Eagle Medium supplemented with 2 mM glutamine, 10 % fetal bovine serum, 20 mg/mL streptomycin, and 20 U/mL penicillin under 5% CO2 at 37°C.
2.2. Cloning, Expression, and Purification of mPD-1
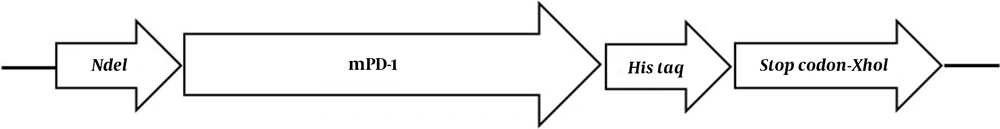
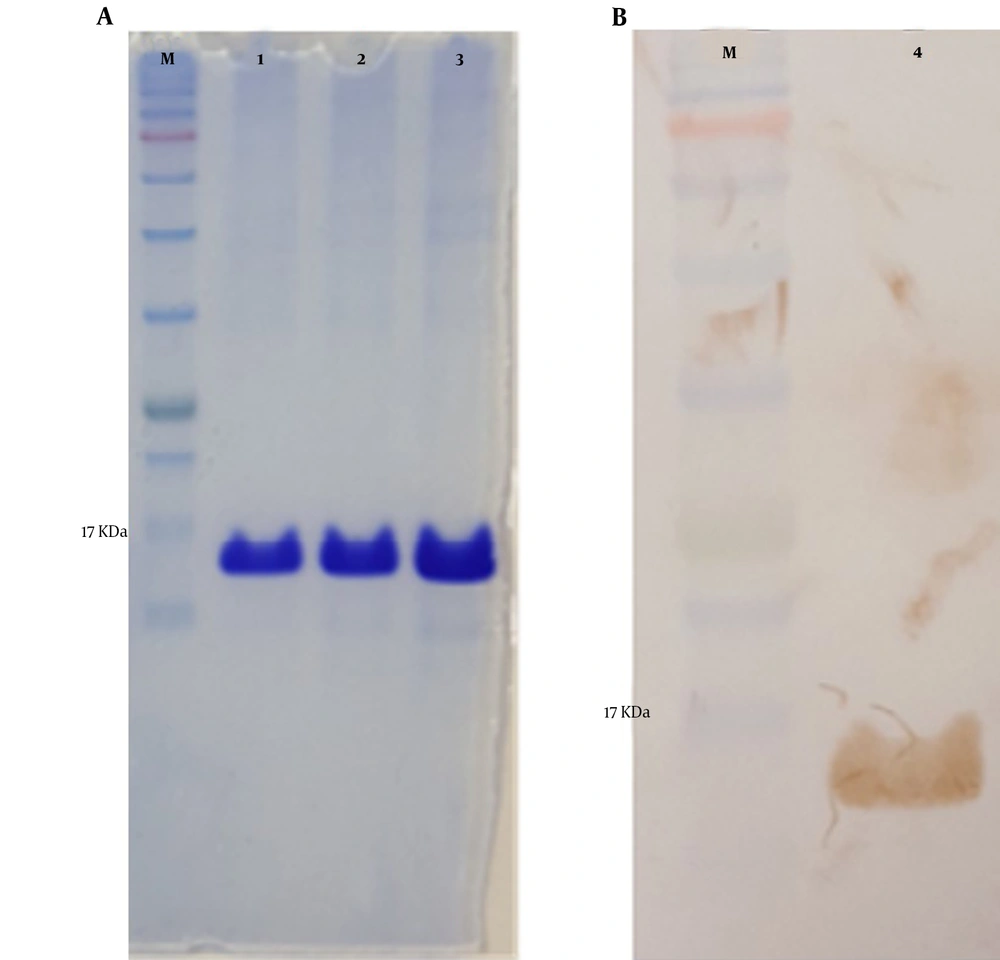
The extracellular mPD-1 coding sequence (residues 21-169) was synthesized and cloned into a pET-26b (+) vector using NdeI and XhoI restriction sites. The recombinant plasmid was transformed into E. coli BL21 (DE3) strain using the heat shock method. For expression, first, a recombinant colony was cultured in an LB medium containing 50 mg/mL kanamycin at 37ºC/200 rpm. For optimization, two different concentrations of isopropyl β- d-1-thiogalactopyranoside (IPTG), when the OD600nm reached 0.6, was added. In addition, two harvesting times (5 h and overnight), and expression temperature (28°C and 37°C), were tested. By optimization, the best protocol for the expression of the protein was found and used for the culture of bacteria within recombinant. After incubation, the cells were centrifuged (8,000 g for 20 min at 4°C) and washed twice in buffer A (8 M urea, 10 mM Tris, 100 mM NaH2PO4, pH 8.0). The pellets were lysed using sonication and centrifuged for 4,000 g / 30 min at 4°C. The supernatant was then applied on nickel affinity column chromatography at a flow rate of 2 ml/min. After washing the column with buffer B (8 M urea,100 mM NaH2PO4, 20 mM imidazole, and 10 mM Tris, pH 6.3), the non-specific proteins were removed. The protein was then eluted with 500 mM imidazole in PBS (pH 7) and refolded using dialysis (12 kDa cutoff) in buffer C (100 mM EDTA in PBS, pH 7.4) after 16 h at 4°C. The protein expression was evaluated by 12% gel SDS-PAGE followed by western blot using rabbit anti-His and goat HRP-conjugated anti-rabbit IgG as primary and secondary antibodies, respectively.
2.3. ELISA
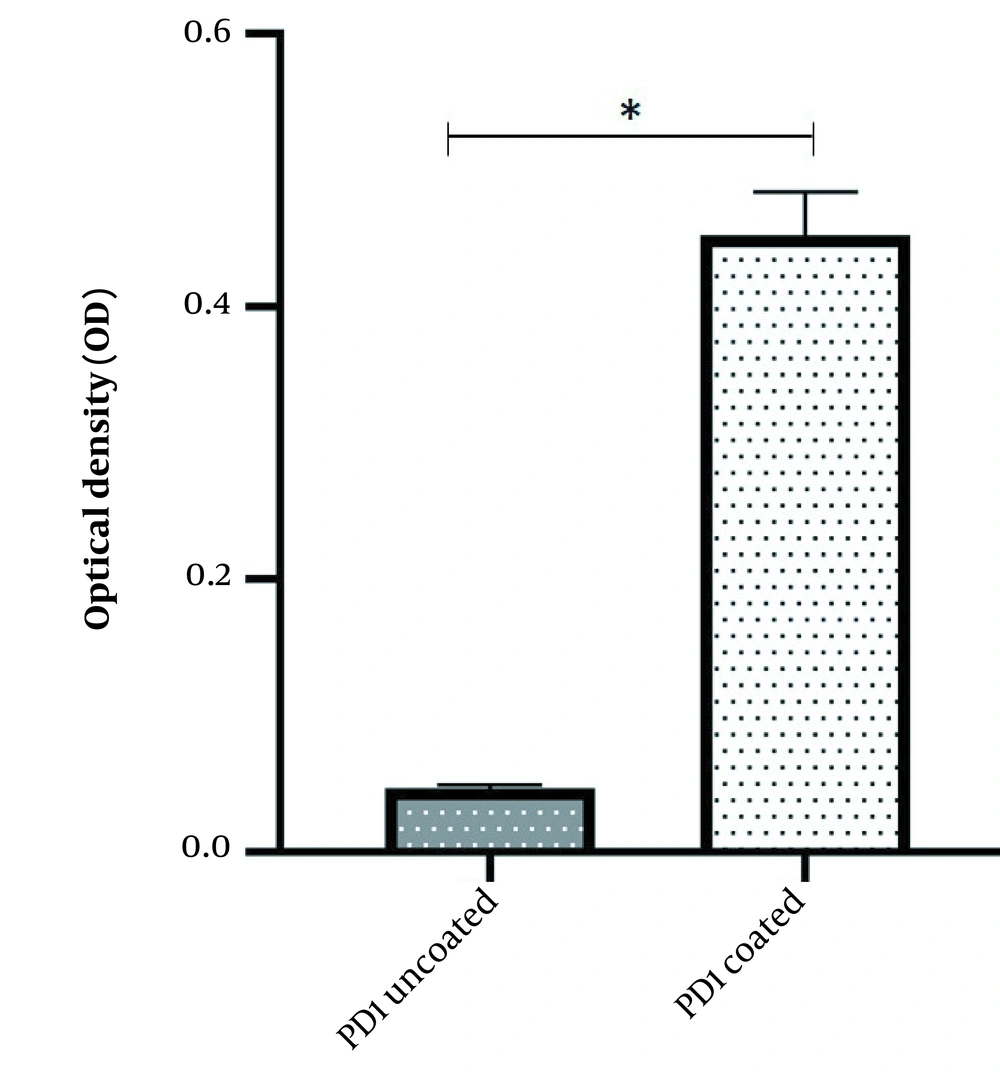
The 0.1 μg per well of mPD-1 recombinant protein was coated on plates and incubated for 1h at room temperature. After three times washing with PBS, the wells were blocked with 4% skim milk overnight at 4°C. Then, 1 µg of the hPD-L1 was added to the wells and incubated for 2 h at room temperature. Next, 100 μL rabbit anti-hPD-L1 (1:2000) and 100 μL goat anti-rabbit HRP were added to the wells after a one-hour interval and three times washing with PBS. The wells were screened by adding 100 μL 3,3′,5,5′-tetramethylbenzidine (TMB) solution. Finally, the enzymatic reaction was stopped using 2N sulfuric acid, and the absorbance was measured using a spectrometer at a wavelength of 450 nm.
2.4. In Vivo Tumor Inhibition
C57BL/6 female mice (4 - 6 weeks) were subcutaneously injected with approximately 3 × 106 TC-1 cells. These mice were utilized as stock. After the tumor establishment, the stock mice were sacrificed, and the tumor was dissected into 3 mm3 pieces and transplanted into the 10 mice. The control group (n = 5) received 150 µL PBS, while the test group (n = 5) received 100 µg mPD-1 subcutaneously around the tumor at one-week intervals. The treatment was continued for eight weeks, and the tumor size and mortality were monitored. The tumor size was measured once a week using a caliper and the following equation (15); V = L × W2 × 0.52, where V is the volume, L is the length, and W is the width of the tumor.
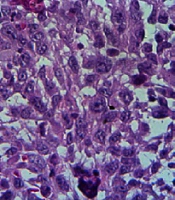
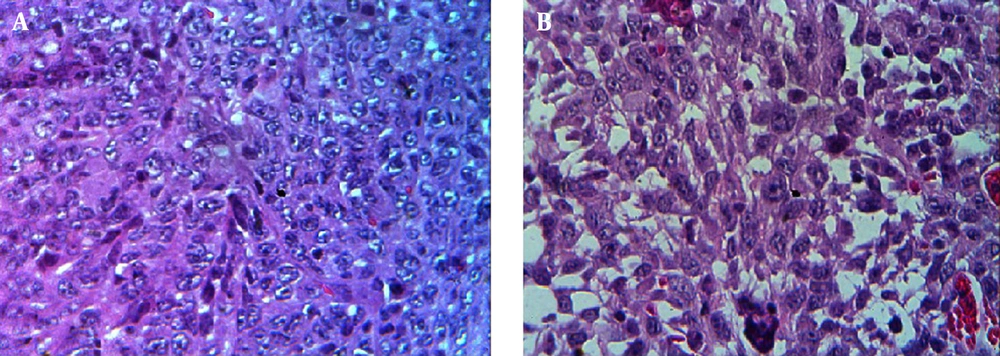
A histological investigation was also employed to evaluate tumor tissue necrosis. The paraffin sections of tumor tissues were deparaffinized, rehydrated, and stained with hematoxylin and eosin for histopathology.
2.5. Statistical Analysis
A two-tailed unpaired t-test was used for statistical analysis, and a p-value less than 0.05 was considered a significant difference between the groups. Curve fittings and regressions were conducted using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA).
3. Results
3.1. mPD-1 Recombinant Expression
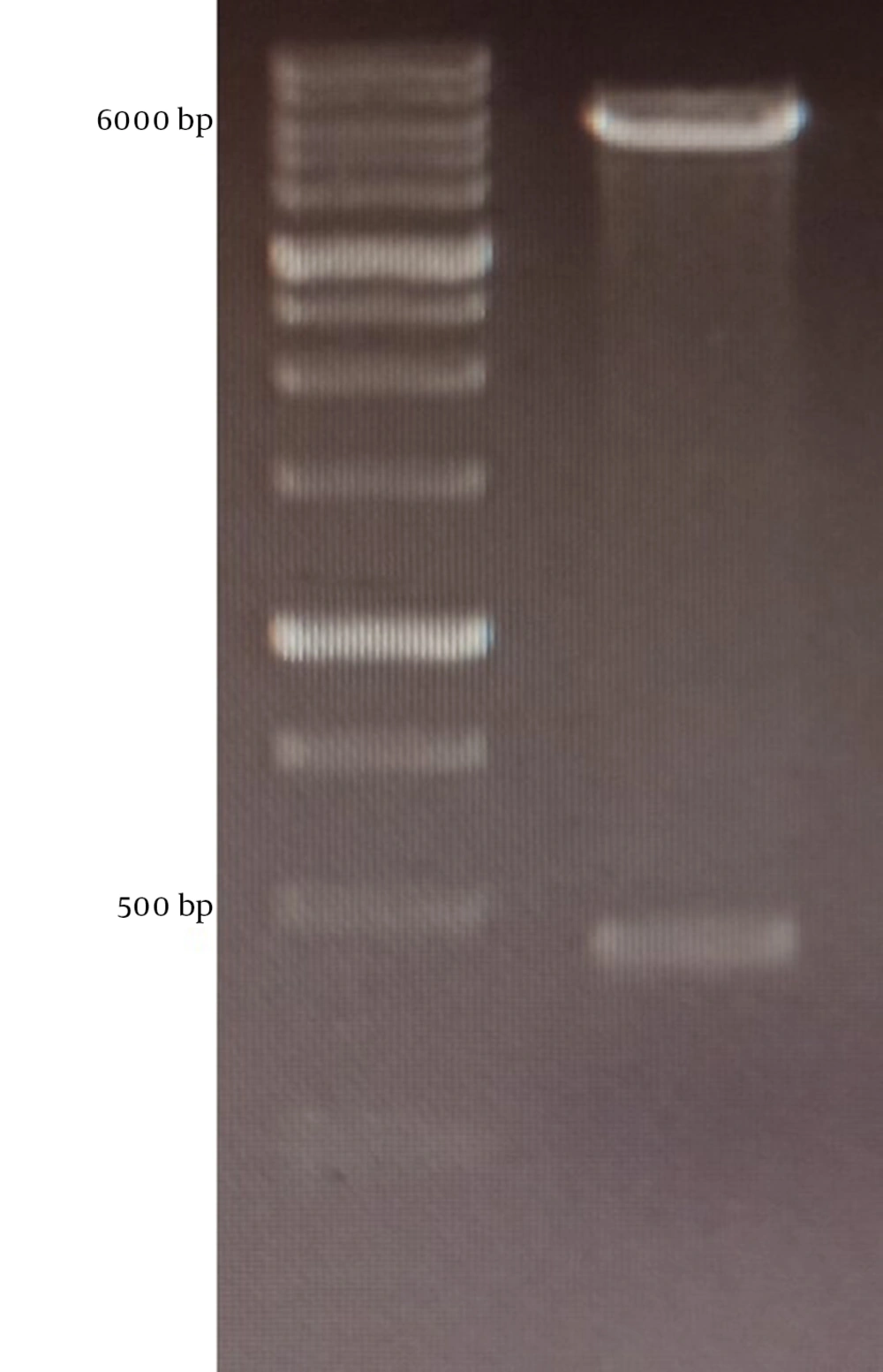
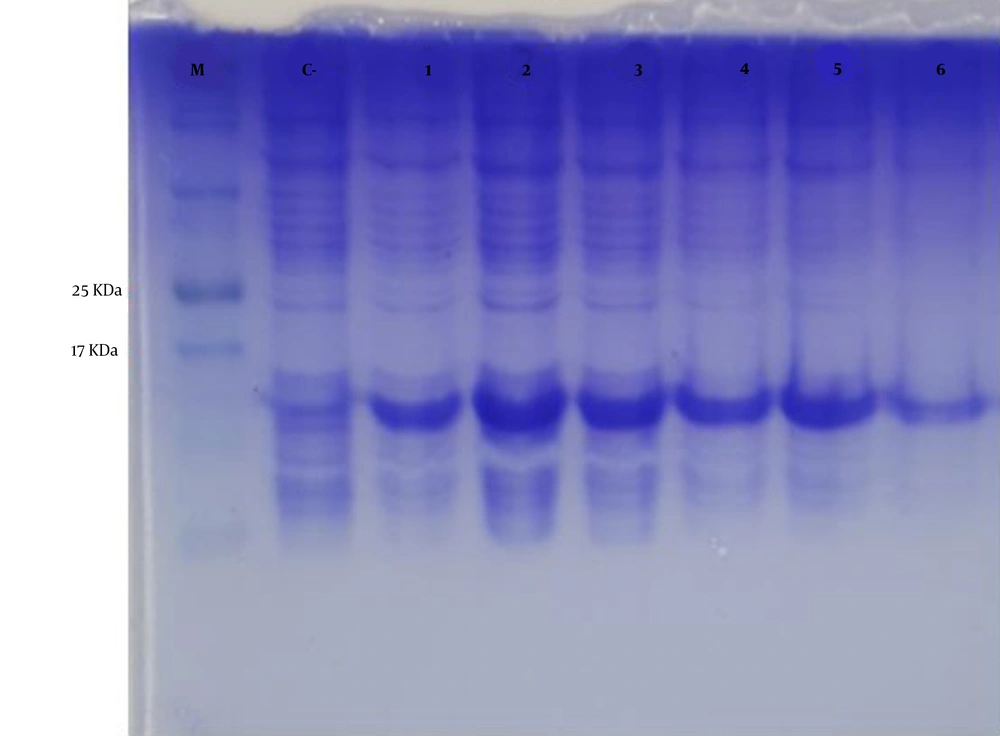
The mPD-1 coding sequence was cloned into pET-26b (+) expression vector using XhoI and NdeI cloning sites (Figure 1) and transformed into E. coli BL21 (DE3). Enzymatic digestion was conducted to confirm the cloning procedure (Figure 2). The expression was optimized based on IPTG concentration, temperature, and incubation time (Figure 3). The best expression occurred after 5 h by adding 0.3 mM IPTG at 28ºC. Recombinant mPD-1 was purified by nickel affinity chromatography under denaturing conditions. After purification, SDS-PAGE and western blot revealed a band at 16.9 kDa, which matches the size of recombinant mPD-1 (Figure 4). The expression yield was about 2 mg/L.
Protein expression optimization. M, protein marker; C-, before IPTG induction; 1, 0.3 mM IPTG, 28°C, overnight incubation; 2, 0.3 mM IPTG, 28°C, 5h incubation; 3, 1 mM IPTG, 28°C, 5 h incubation; 4, 1 mM IPTG, 28°C, overnight incubation; 5, 0.3 mM IPTG, 37°C, overnight incubation; 6, 1 mM IPTG, 37°C, overnight incubation.
3.2. Binding of Recombinant mPD-1 to Human PD-L1
The binding potency of recombinant mPD-1 with human PD-L1 was assessed using ELISA. The outcomes displayed that the recombinant mPD-1 could bind to human PD-L1 (Figure 5).
3.3. Tumor Implantation and In Vivo Study
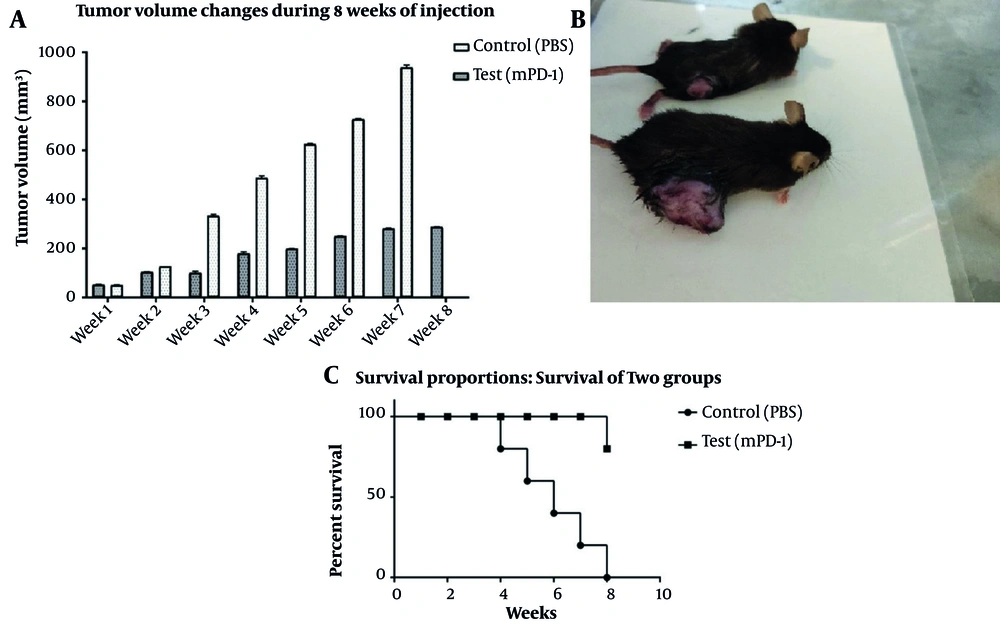
Tumorized C57BL/6 mice treated with mPD-1 for eight weeks at one-week intervals showed a decline in tumor size when compared to the PBS-received group (Figure 6A). After eight weeks, the mean tumor volume in the control and test groups was 938.6 and 281.23 mm3, respectively, which indicated the efficiency of mPD-1 on tumor growth suppression. From weeks 5 to 8, the difference in the tumor size was statistically significant (P < 0.05). The tumor size in two mice was randomly displayed for the control and test groups in Figure 6B. Mice in the treatment group showed a higher survival rate than those in the control group (Figure 6C). From the 4th week, the mice in the control group began to die, and at the end of the 8th week, all mice died, whereas only one mouse died in the test group. Furthermore, hematoxylin and eosin staining exhibited higher necrosis within the tumor in the control group (Figure 7A) compared to the test group (Figure 7B).
Inhibitory effect of tumor growth by recombinant mPD1 in mice model. A, Tumor volume change within eight weeks of tumor challenge (the difference in the 5th to 8th weeks was statistically significant (P < 0.05)). B, Tumor mass in mice receiving recombinant mPD-1 (above) and PBS (below). C, The mice survival rate after the tumor challenge in the test and control groups.
4. Discussion
PD-1 consists of a cytoplasmic domain that binds to the scaffolding and signaling molecules, a transmembrane domain, and a single extracellular domain (16). In the interaction of PD-1 with homogeneous ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), the extracellular domain plays a vital role (17). Myeloid cells, B lymphocytes, and activated T mainly express PD-1 molecules. The interaction of PD-1/PD-L1 or PD-1/PD-L2 prevents B- and T-cell proliferation and inhibits the secretion of cytokines (18). Therefore, targeting the PD-1/PD-1 ligand interaction is considered a promising strategy for generating immunotherapy against various malignancies and some chronic virus infections.
The FDA approved three anti-PD-1 antibodies, including Cemiplimab, used for metastatic cutaneous squamous cell carcinoma treatment, Nivolumab, and Pembrolizumab, both used for metastatic melanoma and non-small cell lung cancer (19). Despite their remarkable clinical outcomes through suppression of the PD-1/PD-L1 interaction, the application of monoclonal antibodies is currently restricted due to improper pharmacokinetics, poor tissue penetration, heterogeneous intra-tumoral distribution, and high cost of production and administration. Some of these advantages are generally attributed to their large size (20-22). Moreover, antibody therapy is accompanied by immune-related adverse events (irAEs) due to unwanted interactions between an antibody and immune system components (23). Therefore, instead of using antibodies, PD-1 or PDL-1 molecules can inhibit them and obtain therapeutic effects to inhibit cancer.
Although the mouse and human PD-L1 proteins have less than 70% sequence similarity, the extracellular IgV domains of both proteins are 100% identical in the amino acid sequence (24). This proposes that the mouse extracellular domain of PD-1 may interact with both mouse and human PD-L1. Here, we have elucidated that the extracellular domain of recombinant mouse PD-1 can interacts with human PD-L1 in vitro. This is appropriate for functional investigations of mPD-1 in the mouse model, which can be extended to human clinical studies. Regarding the PD-1 inhibition by monoclonal antibodies that could strongly improve the therapeutic efficiency of tumors, in this study, we explored whether the recombinant mPD-1 protein has a similar effect (25). The outcomes exhibited that the tumor growth was efficiently suppressed in the mice treated with mPD-1 recombinant protein.
PD-1 and its ligand interactions, regulation, and expression pattern enrich our knowledge about how co-inhibitory and costimulatory molecules have been employed via cancer cells for immune evasion. Recently, the soluble forms of PD-L1 (sPD-L1) and PD-1 (sPD-1) have been detected in the plasma of cancer patients, and subsequently, several studies have been conducted (26, 27). Generally, these studies have been focused on prognostic and predictive values. Numerous investigations have shown that raised expression of sPD-L1 and sPD-1 may predict the worst prognosis in cancer patients (28, 29). Soluble forms may be expressed to preserve immune evasion and peripheral self-tolerance (30). An increase in the expression of sPD-L1 or sPD-1 can disrupt this balance and improve T-cell activation by interrupting the PD-1/PD-L1 interaction. These kinds of effects are detected in clinical investigations. The level of each molecule can decrease or increase via therapeutic intervention. For example, a rise in sPD-1 expression at the post-therapeutic stage is related to enhanced results. Moreover, an increase in sPD-L1 level is linked with the worst consequence and reduced efficiency of anti-PD-1 monoclonal antibodies (31, 32). However, the increased level of sPD-1 was related to the enhanced efficiency of Nivolumab as an anti-PD-1 monoclonal antibody (33). Since the interaction between sPD-1 and its ligands can disrupt the PD-1/PD-L1 pathway, sPD-1 may be employed as a therapeutic approach for disrupting PD-L1 interactions in the same style as monoclonal antibodies. Several in vitro and in vivo investigations have elucidated the effective stimulation of antitumor immunity and reduction in cancer growth that occurred with an elevated sPD-1 level in the tumor microenvironment (13).
Similar to our study, in 2008, Zhang et al. showed that the administration of PD-1 protein could prevent tumor growth in mouse models. In that study, human PD-1 protein was used, while we used the mouse type of this protein. The results of Zhang's study are in high agreement with the results of the present study and emphasize the ability of PD-1 protein as a tumor therapeutic candidate (34). Mouse PD-1 shares 64% sequence identity with its human ortholog, and mouse PD-1 binds in vitro to both human and mouse PD-L1 (35). The main obstacle in appraising anti-PD-1 therapeutics in syngeneic mouse tumor models is the low homology (61% identity) among the extracellular domains of mouse and human PD-1 (36). Thus, mouse PD-1 can be a promising candidate for studying PD-1 inter-species differences in humans and mice. Interspecies investigations propose an incompatibility among some human and mouse proteins (37). It is unidentified whether inter-species differences in the PD-L1 structure and sequence would allow for mouse-human cross-applications (24). Recently, the recombinant mouse PD-1–human Fc chimera fusion protein has been employed to study the PD-1 blockade effect on the primary and recall antibody responses and cross-reaction (38).
On the other hand, prior investigations have revealed that glycosylation is not essential for PD-1 binding activity (39). Our outcomes also showed that bacterially expressed recombinant mPD-1 protein could interact with PD-L1 in vitro, and this interaction can block the PD-1/PD-L1 pathway in vivo. This bacterial expression system is available and cheap and can be utilized as a low-cost medication for cancer therapy (40).
4.1. Conclusions
The effect of PD-1 protein was shown in inhibiting cancerous mass in tumor-bearing mice. Considering that mouse PD-1 protein can identify human PDL-1 protein, the results can be generalized to humans. Our study suggests that the recombinant mPD-1 protein can be a potential candidate for tumor therapy.