1. Background
Remaining as a critical global burden on public health, diabetes mellitus (DM) is a chronic multifactorial disorder that is mainly characterized by hyperglycemia and impaired metabolism of carbohydrates, lipids, and proteins owing to an unstable insulin secretory state, development of resistance to the secreted insulin or both (1). Cannabis, also referred to as "marijuana," is one of the most frequently consumed psychoactive drugs worldwide, which is growingly administered in developing countries, including Iran (2). While it has been demonstrated that consumption of marijuana is together with an enhancement in the daily calorie intake (3), marijuana consumption has been shown to be associated with a lower body mass index (BMI) (4) and the probability of DM occurrence (5). Whereas the underlying mechanism for this paradoxical event is unknown, the study performed by the National Health and Nutrition Examination Survey (NHANES) on 4657 habitual consumers of marijuana demonstrated that fasting blood glucose (FBS), as well as insulin level and development of insulin resistance were significantly lower than that of nonusers (6). As Δ9-tetrahydrocannabinol (Δ9-THC) and cannabidiol (CBD) are the main bioactive components of cannabis, observed therapeutic effects of cannabis may also be related to the same compounds.
Δ9-THC is the psychoactive component of the cannabis plant which acts through both the cannabinoid 1 and 2 (CB1 and CB2) receptors (7). Through this, Δ9-THC can modulate physiological activities of the gastrointestinal tract, liver, cardiovascular system, pain sensation, and regulation of the neurotransmitter's secretion in the central nervous system (CNS) (8). It has been shown that Δ9-THC can also improve the adipocytes' sensitivity to the secreted insulin. Moreover, natural extracts containing Δ9-THC have been shown to effectively reduce the triacylglycerol (TAG) reservoir and improve glucose uptake in the 3T3-L1 insulin-resistant cell line in a dose-dependent manner attributed to the enhanced secretion of tumor necrosis factor-alpha (TNF-α) by these cells. Moreover, Δ9-THC administration has been shown to enhance the expression of the glucose transporter type 4 (GLUT4) and insulin receptor substrate 1 and 2 (IRS-2) genes, both of which are central to the regulation of insulin's activated signaling pathway (9).
Contrary to the Δ9-THC, CBD is the most abundant non-psychoactive phyto-cannabinoid found in cannabis, which is incapable of activating CB1 and CB2 receptors in the CNS. Considering its non-psychoactive nature, CBD is routinely administered for the treatment of psychosis (10) and the misusage of cannabis (11). Based on the recent findings, specific receptors involved in the medical activities of CBD consists of the orphan G-protein–coupled receptor-55 (GPR55), the transient receptor potential vanilloid 1 (TRPV1) receptor, α1-adrenoceptors, µ opioid receptors, and the adenosine transporter and serotonin-1A receptors (7). CBD can also activate physiologic reactions associated with peroxisome proliferator–activated receptor γ (PPAR γ) activation (12). Moreover, according to recent in vivo studies, CBD demonstrates a range of protective effects against hyperglycemia-induced destructive events in rodents, owing to its anti-inflammatory and anti-oxidative behavior (13, 14). In this context, using ob/ob transgenic mice, Stanley et al. demonstrated that administration of a 3 mg/kg CBD regimen during a four-week treatment period could result in a 55% enhancement in HDL-C plasma level and more than 25% reduction in total cholesterol levels (14). Moreover, the same concentration of CBD could meaningfully reduce liver triglycerides (TG) while enhancing liver glycogen synthesis and adiponectin secretion (15).
Consequently, based on the above-mentioned studies, it is rational to apply a mixture of Δ9-THC and CBD as a supplementary regimen, along with the main anti-diabetic medications, to improve DM patients' responses to therapy.
2. Objectives
In the present study, we investigated the efficacy and safety of a sublingual spray, CBDEX1® (Yas Daru, Tehran, Iran), comprising a combination of CBD and Δ9-THC in a 10:1 ratio (delivering 100 µg CBD and 10 µg Δ9-THC per puff), administered twice per day and two puffs each time, over an eight-week treatment period, on glycemic state and lipid profiles of patients with type II DM.
3. Methods
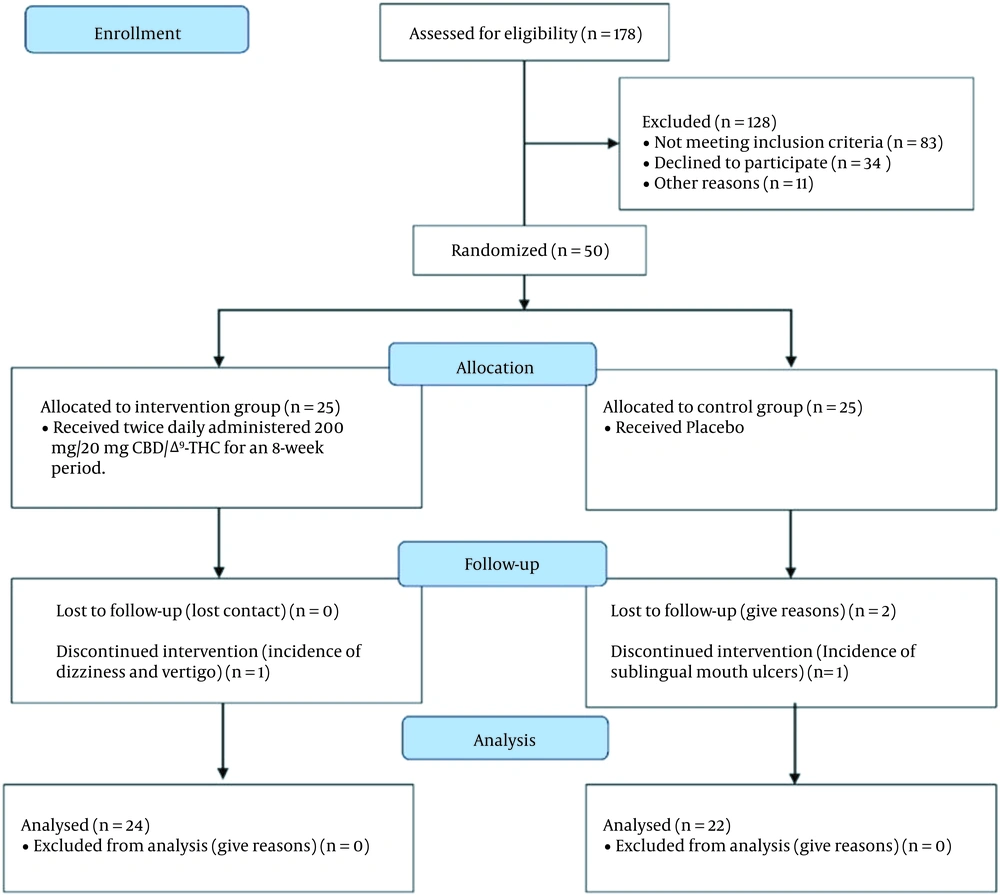
Participants in the present randomized, double-blind, placebo-controlled, phase I clinical trial were recruited from diabetes care-seeking patients who attended the endocrinology department of the Imam Hossein hospital, affiliated with Shahid Beheshti University of Medical Sciences, Tehran, Iran, between January 2021 and April 2021. The study was approved by the ethics committee of the Shahid Beheshti University of Medical Sciences (IR.SBMU.PHARMACY.REC.1399.302) and registered in the Iranian clinical trial registry (IRCT20121021011192N10). Seventy-eight patients, fulfilling the inclusion criteria characteristics of the study, were initially invited to participate in the study and enrolled in a briefing session to become familiar with the objectives and rationale of the study. In the next step, the acceptance or refusal statement to the invitation was recorded for all invited patients. For refusals, the underlying reason was also recorded. In the decision-making session, twenty-eight invited patients refused to be enrolled in the study. Hence, a total of 25 eligible patients remained enrolled in each arm of the study.
The primary outcome of the present study was to assess the efficacy of the twice daily administered 100 µg/10 µg CBD/Δ9-THC supplementary therapy in controlling the glycemic state and lipid profile of type II diabetic patients compared to the placebo-receiving group. The secondary and tertiary outcomes were to evaluate the safety and quality of life in mentioned patients. Fasting blood glucose (FBS), Postprandial glucose (PPG), and hemoglobin A1C (Hg A1C) were used as glycemic outcomes. Also, the homeostasis model assessment of Insulin resistance (HOMA-IR) and beta cell function (HOMAβ) were applied as indexes to evaluate insulin resistance. Cholesterol, LDL-cholesterol, and triglyceride have been used as lipid outcomes in the present trial.
Safety assessment included evaluating patients for adverse and severe adverse effects, monitoring vital signs, and performing before and after treatment laboratory tests and electrocardiograms. In the present study, causality assessment between the medication or placebo and the suspected reaction was determined by using the Naranjo scale. According to mentioned criteria, adverse drug reactions (ADRs) are analyzed based on a ten-question questionnaire in which each question is given a score of +2, +1, 0, or -1. If the total score is more than nine, its label is a definite ADR (16). In this trial, we have considered any definite reactions as ADRs.
For the trial, the placebo was prepared with the same appearance as a sublingual spray of cannabidiol /delta 9-tetrahydrocannabidiol and precisely according to the main drug formulation, but without the active ingredient. The inclusion criteria of the study consisted of a confirmed diagnosis status of type II DM, a minimum age of eighteen years old or older, an Hb A1C value of ≤ 9%, a plasma HDL-C concentration of ≤ 50 mg/dL in females and ≤ 46.5 mg/dL in males, and a plasma TGs level of ≤ 800 mg/dL. Moreover, in the cases of non-insulin glucose-lowering agent's consumption (e.g., metformin, sulfonylurea, dipeptidyl peptidase-4 inhibitors, or glucagon-like peptide 1), the dosage had to be constant for at least three months before inclusion to the study. Similarly, enrolled patients had to receive a constant dosage of statins or other lipid-lowering drugs at least four weeks before inclusion in the study period. Enrolled patients also had to have a constant diet regimen or exercise schedule from 4 weeks prior to the initiation of the study up to the end of the study course. The most important exclusion criteria consisted of recent or ongoing consumption of forbidden therapeutic agents comprising insulin, omega-3 fatty acids, fibrates, thiazolidinediones, and α-glucosidase inhibitors; recent or ongoing consumption of cannabis or its related products; familial dyslipidemia disorders as well as the existence of critical cardiovascular, renal or hepatic comorbidities.
Following the screening session (visit 1), a routine laboratory blood chemistry panel (comprising liver enzymes) was performed at the treatment randomization step (visit 2) and considered as the patient's baseline values. Eight weeks after the initiation of the study, patients were again subjected to the laboratory blood chemistry panel test (visit 3) and considered as the patient's post-treatment values. Safety evaluation was performed throughout the study period, and a safety follow-up visit was carried out a week following the completion of the study or withdrawal of the patients (visit 4). Medication consisted of a sublingual spray, CBDEX10®, composed of a combination of CBD and Δ9-THC in a 10:1 ratio (delivering 100 µg CBD and 10 µg Δ9-THC per puff), administered twice per day and two puffs each time to the patients. Medication was administered in the fasted state to the patients (30 min before breakfast and dinner) with an approximately 12 h intervals between two administrations. An online statistical computing web program was used to randomize the participant placement (http://www.graphpad.com/quickcalcs/randomize1.cfm). The "patient interview method" has been used to assess adherence. Interviewing patients by physicians is typically a friendly, low-cost technique to evaluate patient adherence (17). During the trial, evaluators called participants and were asked to estimate their medication-taking behavior, namely, the percentage of dose they may miss within a designated period or the frequency with that they cannot follow the medication regime.
For calculation of the sample size, we considered previous studies demonstrating that cannabis can induce a 32 mg/dL reduction in the FBS level of the treated group with a standard deviation (±) of 4.6. Considering a clinically significant difference level of 13 mg/dL (18), a significance level of 0.05, a power of 80%, and a two-sided analysis, the sample size of each group was calculated to be equal to 25.
All statistical analyses were performed two-sided using SPSS software version 28.0 (IBM, Armonk, NY, USA). The Shapiro-Wilkins normality test was performed for each categorical data prior to the performance of desired statistical tests. Primary and secondary endpoints of the study were evaluated utilizing the analysis of the co-variances (ANCOVA) of the changes from baseline values to the end of the treatment period for the related parameter, considering the baseline value of the parameter as the covariate and treatment as the factor. Demographic data and safety profile of patients were also analyzed by fisher's exact test. Differences were considered to be statistically significant for P-values lower than 0.05.
4. Results
A total of 50 patients participated in the study: twenty-five were randomly allocated to the twice-daily 200 mg/20 mg CBD/Δ9-THC (supplementary group) and 25 to the placebo group, as demonstrated in Figure 1. Analysis was performed on anthropometric, clinical, and demographic data obtained from those who could successfully finish the 8-week clinical study. The demographic data and the clinical properties of the studied patients at the baseline level are reported in Table 1. No statistically significant differences were observable regarding the demographic properties of patients between the two studied groups.
| Variables | CBD/Δ9-THC (10:1) Group (n = 24) | Placebo Group (n = 22) | P-Value |
|---|---|---|---|
| Age, y | 55.7 ± 1.7 | 53.2 ± 1.3 | 0.26 |
| Gender (males) | 6 (33.34) | 5 (27.8) | 0.99 |
| Body mass index, kg/m2 | 30.7 ± 0.8 | 31.2 ± 0.7 | 0.64 |
| Current smoker | 3 (16.67) | 2 (11.2) | 0.99 |
| Microvascular complications | 1 (5.6) | 1 (5.6) | 1 |
| Hypertension | 0 (0) | 1 (5.6) | 0.99 |
| Fasting glucose, mg/dL | 144.9 ± 17.9 | 139.2 ± 16.5 | 0.27 |
| Cholesterol, mg/dL | 159.2 ± 10.2 | 145.8 ± 9.9 | 0.35 |
| Triglyceride, mg/dL | 169.8 ± 21.4 | 134.2 ± 13.7 | 0.16 |
| HDL-cholesterol, mg/dL | 41.06 ± 1.3 | 42.3 ± 1.6 | 0.57 |
| LDL-cholesterol, mg/dL | 98.53 ± 8.2 | 86.9 ± 8.8 | 0.34 |
| Creatinine, mg/dL | 0.96 ± 0.08 | 0.92 ± 0.06 | 0.2 |
| Hb A1C, % | 7.94 ± 0.34 | 7.41 ± 0.16 | 0.18 |
| Insulin baseline (mIU/L) | 26.27 ± 3.60 | 24.2 ± 3.70 | 0.08 |
| HOMA2-IR | 3.68 ± 0.65 | 3.36 ± 0.66 | 0.10 |
| HOMA2β | 91.2 ± 9.4 | 92.5 ± 9.3 | 0.80 |
a Values are expressed as No. (%) or mean ± SD.
As presented in Table 2, just similar to the lipid profile, the plasma values of FBS, Hb A1C, and 2-h OGTT test in the supplement group were significantly lower than those observed in the placebo group after the 8-week treatment period (all P < 0.05). Compared to their baseline values, FBS, Hb A1C, and 2-h OGTT test values were significantly declined only in the supplement group (all P < 0.05), while those of the placebo group were only insignificantly increased. In parallel with these findings, a statistically significant decline in insulin secretion and HOMA2-IR values was recorded in the supplemented group compared to the placebo group between baseline and the end of the eight-week treatment period (ETD = -5.21 mIU/L, P < 0.001 and ETD = -0.77; P < 0.001 respectively). Despite this, no meaningful differences in HOMA2β cell function values were observed between the supplement-treated and placebo arm (ETD = -0.22, P = 0.7).
| CBD/Δ9-THC (10:1) (n = 24) | Placebo (n = 22) | CBD/Δ9-THC (10:1) vs Placebo P-Value | |||||
|---|---|---|---|---|---|---|---|
| After 2 Months | Change from Baseline, % | P-Value | After 2 Months | Change from Baseline, % | P-Value | ||
| Cholesterol, mg/dL | 149.3 ± 5.8 | -8.0 | < 0.001 | 152.8 ± 7.08 | +4.65 | 0.023 | < 0.001 |
| Triglyceride, mg/dL | 143.8 ± 10.0 | -15.4 | 0.032 | 132.36 ± 7.3 | -1.04 | 0.433 | < 0.001 |
| HDL-cholesterol, mg/dL | 43.3 ± 0.88 | +4.08 | 0.083 | 44.08 ± 5.7 | 0 | 1 | 0.083 |
| LDL-cholesterol, mg/dL | 98.5 ± 4.8 | -4.70 | 0.014 | 92.3 ± 5.8 | +2.67 | 0.133 | < 0.001 |
As depicted in Table 3, the plasma values recorded for T-CHOL, TG, and LDL-C in the treatment group were significantly lower than those observed in the control group after the eight-week treatment period (all P < 0.05). Nevertheless, no statistically significant difference was observable between the mean plasma values of HDL-C between the two groups (P = 0.084). Moreover, compared to their baseline values, T-CHOL, TG, and LDL-C levels were significantly declined only in the treatment group (all P < 0.05), while values of HDL-C were not meaningfully changed in both groups at the end of the eight-week treatment period (P = 0.084 and P = 1 for supplement group and placebo group respectively). In this context, reduction values for T-CHOL, TG, and LDL-C levels were 8.0%, 15.4%, and 4.7%, respectively, in the treatment group. Noteworthy, a statistically significant increase in T-CHOL level was reported in the control group at the end of the studied period (+4.65 %, P = 0.023). While the mean plasma value of HDL-C was not changed before and after treatment in the control group, LDL-C was insignificantly increased by +2.67 % in the same group at the end of the study (P = 0.113). Since there were no statistically significant changes between the two groups at the baseline, these outcomes at the end of the eight-week treatment period represent the positive effects of the adjunctive regimen in controlling the lipid profile of patients with type II diabetes.
| CBD/Δ9-THC (10:1) (n = 24) | Placebo (n = 22) | CBD/Δ9-THC (10:1) vs Placebo P-Value | |||||
|---|---|---|---|---|---|---|---|
| After 2 Months | Change from Baseline, % | P-Value | After 2 Months | Change from Baseline, % | P-Value | ||
| Fasting glucose, mg/dL | 133.7 ± 9.7 | -7.5 | 0.0125 | 140.0 ± 9.8 | +0.5 | 0.84 | < 0.001 |
| Hb A1C, % | 7.8 ± 0.24 | -8.5 | < 0.001 | 7.35 ± 0.15 | +1.3 | 0.075 | < 0.001 |
| Baseline insulin mIU/L | 22.53 | -16.6 | < 0.001 | 25.67 ± 12.2 | +5.7 | 0.59 | < 0.001 |
| HOMA2-IR | 3.12 ± 0.12 | -17.94 | < 0.001 | 3.57 ± 0.13 | +5.89 | < 0.001 | < 0.001 |
| HOMA2β | 93.8 ± 6.1 | +2.78 | 0.3565 | 95.4 ± 9.2 | +3 | 0.30 | 0.7 |
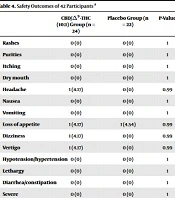
The adverse effects of administering the twice-daily supplement regimen are depicted in Table 4. Overall, the mentioned adjunctive regimen was well tolerated. Except for one case with simultaneous signs of vertigo and dizziness who was withdrawn from the study, no other severe adverse effects were observed. Noteworthy, a case of sublingual oral sore occurred in the control group, which resulted in the exclusion of the patient from the study. Finally, there were no statistically significant differences in reported adverse effects between the two groups (for all, P > 0.05).
| CBD/Δ9-THC (10:1) Group (n = 24) | Placebo Group (n = 22) | P-Value | |
|---|---|---|---|
| Rashes | 0 (0) | 0 (0) | 1 |
| Purities | 0 (0) | 0 (0) | 1 |
| Itching | 0 (0) | 0 (0) | 1 |
| Dry mouth | 0 (0) | 0 (0) | 1 |
| Headache | 1 (4.17) | 0 (0) | 0.99 |
| Nausea | 0 (0) | 0 (0) | 1 |
| Vomiting | 0 (0) | 0 (0) | 1 |
| Loss of appetite | 1 (4.17) | 1 (4.54) | 0.99 |
| Dizziness | 1 (4.17) | 0 (0) | 0.99 |
| Vertigo | 1 (4.17) | 0 (0) | 0.99 |
| Hypotension/hypertension | 0 (0) | 0 (0) | 1 |
| Lethargy | 0 (0) | 0 (0) | 1 |
| Diarrhea/constipation | 0 (0) | 0 (0) | 1 |
| Severe hypoglycemia | 0 (0) | 0 (0) | 1 |
| Blurred vision | 0 (0) | 0 (0) | 1 |
| Weight gain/loss | 0 (0) | 0 (0) | 1 |
| GI distress | 0 (0) | 0 (0) | 1 |
| Other | 2 (8.34) | 1 (4.54) | 0.99 |
a Values are expressed as No. (%).
5. Discussion
In the present study, we demonstrated that sublingual administration of the CBDEX1® sublingual spray, two puffs twice daily through an eight-week treatment period, could effectively improve the patient's lipid profile and glucose tolerance. Moreover, the mentioned regimen could also improve insulin sensitivity, evident from a meaningful enhancement in the HOMA2 values. Consistently, the study performed by Penner et al. demonstrated that insulin level ,HOMA-IR index, and waist circumference is lower in marijuana users than control ones (6). Moreover, investigating the data released by the Third National Health and Nutrition Examination Survey (NHANES III) revealed that the incidence rate of DM was significantly lower among marijuana consumers compared to the non-consumer group (4). Contrary to our findings, however, the pilot study performed by Jadoon et al. showed that a 1:1 and 20:1 combination of CBD and cannabivarin (CBV), a homolog of Δ9-THC, could not effectively reduce fasting blood glucose level and lipid content of DM patients after a 13-week treatment period. As the administration of each drug alone could somehow affect several study's endpoint values, the authors concluded that the combination administered at the studied ratio might have an antagonizing effect on each other (18). The first reason underlying this discrepancy is that compared to the concentrations of Δ9-THC and CBD applied in previously performed clinical studies with meaningful positive outcomes, the one applied in Jadoon and colleague’s study was extremely low, making extrapolation of results to the higher concentration somehow difficult and doubtful (19). Another important reason for this discrepancy may be the differences in Δ9-THC and CBD’s root of administration. Owing to their highly lipophilic nature, Δ9-THC, and CBD are routinely formulated as oil preparation and demonstrate a very poor oral bioavailability in humans (approximately equal to 6%) (20).
Moreover, orally administered Δ9-THC or CBD formulation is extensively metabolized in the liver following adsorption from the gastrointestinal tract due to CYP3A4 and CYP2C19 metabolizing enzymes activity (20, 21). Consequently, the very low orally absorbed amount of Δ9-THC or CBD will be immediately subjected to a large first-pass effect, only permitting an extremely low amount of intact Δ9-THC or CBD to reach the bloodstream. In contrast, sublingual administration of Δ9-THC and CBD can significantly improve the bioavailability of the drug (reaching a value of 20% (20)), enhancing peak plasma concentration (Cmax) of Δ9-THC and CBD and the time required for reaching it (Tmax) (22). In this context, the large part of the sublingually administered Δ9-THC and CBD combination can successfully bypass the first pass effect and other complications associated with the oral route administration, providing a much higher Cmax and Tmax values and consequently, a more rapid onset of action for Δ9-THC and CBD in sublingual form.
Although the exact mechanism underlying the positive modulatory activity of cannabinoids on peripheral metabolism has not yet been fully understood, the activity of these compounds on CB receptors of peripheral organs may somehow explicate the answer. In this context, it has been shown that administration of rimonabant, a CB1 antagonist, was together with a significant improvement in the sensitivity of normal mice to insulin, proposing that adiponectin may be the responsible molecule in the improvement of sensitivity to insulin (23). This finding has also been established in human studies. Moreover, in another clinical trial, the administration of rimonabant was in a statistically significant enhancement in the adiponectin plasma concentration, weight loss, and a meaningful reduction in circumference value (24). Additionally, the administration of cannabis to obese rats was with a meaningful increase in pancreas weight which is highly suggestive of its protective effect on beta cells viability and functionality (25). Considering that the CB1 receptor knockout mice are also persistent in diet-induced obesity, the role of the CB1 receptor in metabolism and obesity becomes much bolded (26). Since both Δ9-THC and CBD can induce an antagonistic effect on cannabinoid receptors, it may be concluded that the observed protective effects of both compounds may be partly mediated through the enhancement of secretion of adiponectin.
As mentioned earlier, CBD is considered to be a CB1 receptor inverse agonist, resulting in the reduction of CB1 receptor constitutional functioning or attenuation of the endocannabinoid tone. Moreover, micromolar concentrations of CBD could show some inhibitory effects on fatty acid amide hydrolase enzyme activity and hence, can promote the accumulation of arachidonoyl ethanolamine (AEA), a well-known agonist for CB receptors. Moreover, the same concentrations from CBD could also effectively enhance 2-AG levels in humans, a selective CB1 receptor agonist (27). Hence, in theory, high enough concentrations of CBD may also induce beneficial lipid-lowering effects observed with rimonabant. In this context, administering 800 mcg/day of CBD in schizophrenic patients could effectively enhance plasma AEA concentrations and improve the patients' clinical signs (9). Considering the much higher bioavailability of sublingually administered CBD, the 400 µg/day concentration from CBD may be the optimum tolerable, safe, and effective concentration for application in the clinic.
Consistent with our findings, numerous animal studies have shown glucose-controlling and lipid-lowering activities for CBD. For instance, in the study by Lehmann et al., the pretreatment of mice with CBD could delay the onset of diabetic signs (including hyperglycemia) following the induction of type I diabetes by pancreas inflammation (28). Despite this, the study performed by Rajesh et al. demonstrated that administration of CBD could not significantly improve blood glucose levels in streptozotocin (STZ) induced diabetic mice, which may be due to the time of administration of CBD which was one-week following induction of diabetes and development of hyperglycemia, meaning that the large part of the functional beta cells may have become destroyed (13). On another side, McKillop et al. demonstrated that administration of a synthetic derivate of CBD could lower T-CHOL and TG up to 17% and 19%, respectively, while enhancing HDL-C by 19% in STZ-induced diabetic mice (29). Similarly, Zorzenon et al. demonstrated a significant improvement in lipid profile (including TG, T-CHOL, LDL-C, and HDL-C) of diabetic mice undergoing cerebral ischemia, treated with CBD and proposed it as an effective pharmacological agent in preventing metabolic dysfunction development in diabetic patients with cerebral ischemia (30).
The 100 µg/10 µg CBD/Δ9-THC 2puffs twice per day sublingually administered regimen of the present study was well tolerated by patients. Except for one case with vertigo and dizziness, no other serious adverse effects were reported. Consistently, in phase I clinical trial, evaluating the safety, tolerability, and pharmacokinetics of a CBD oral solution, most patients could tolerate up to 6000 mg/day of CBD, and none demonstrated severe or serious adverse effects at all (31). Based on these observations, the combination of CBD/Δ9-THC regimen could be a new therapeutic regimen for controlling the lipid profile and glycemic state of DM patients. Despite this, one of the main limitations of the present study was the low number of the studied population. Moreover, the lack of confirmatory laboratory tests, including measurement of the levels of adiponectin, resistin, GIP, Apo A, etc., is another limitation of the present study. Finally, for a more precise analysis of results, restriction or sub-classification of patients according to their receiving anti-diabetic regimen will be much more appreciated.