1. Background
Hair follicles (HFs) undergo life-long cyclical transformations (1). The hair cycle process proceeds in three stages: Anagen (hair growth), catagen (detachment from the blood vessels and dermal papilla), and telogen (hair detached and resting on the skin) (2). The hair cycle is accomplished by repeated activation and suppression of HF stem cells, regulating proliferation and apoptosis. Hair follicle cycle abnormalities, like alopecia, underlie many human hair growth disorders (3).
Androgenetic alopecia (AGA), a common alopecia, affects both sexes, especially older men (4). The main characteristics of AGA are HF miniaturization and hair diameter reduction by repeated hair cycles with shortened anagen phase (5). Dihydrotestosterone (DHT) is the main mediator of AGA, which is converted from testosterone by 5α-reductase type 2. Dihydrotestosterone binds to the androgen receptor (AR) of the dermal papillae and moves into the nucleus, resulting in new hair formation by inducing apoptosis, suppressing proliferation, and sustaining telogen. Dihydrotestosterone disrupts the hair cycle by prematuring the catagen phase and extending the telogen phase (6).
One of the critical pathways in hair growth cycling is Wnt/β-catenin signaling which is critical for HFs regeneration and growth of the hair shaft (7). The molecular cross-talk between androgens and Wnt signaling in dermal papilla cells was described with the activation of glycogen synthase kinase-3β (GSK3β) (8). Glycogen synthase kinase-3β which is involved in dephosphorylation mediated by androgens appears to contribute to the inhibition of Wnt/β-catenin signaling, resulting in β-catenin degradation (9). Androgen complex also upregulated paracrine factors such as transforming growth factor-beta 1 (TGF-β1), interleukin-6 (IL-6), which is involved in keratinocyte growth, and dickkopf-a (DKK-1), which is an inhibitor and blockers of binding Wnt to the frizzled receptor (10).
Finasteride, a 5α-reductase type 2 inhibitor, acts on anti-androgen by reducing DHT in the prostate and scalp (11, 12). Several clinical studies have shown that finasteride causes hair loss progression and increases hair growth (13, 14). However, natural products that can reduce side effects are being studied as alternative treatments due to the known side effects of finasteride, such as male dysfunction and birth defects (15, 16).
Stauntonia hexaphylla (Thunb.) Decne., which belongs to the Lardizabalaceae family and is mainly distributed in Korea, Japan, Taiwan, and China. According to traditional Chinese medicine, the roots, leaves, and fruits of S. hexaphylla are traditionally used to treat conventional arthritis and neuralgia. It has also been used as a folk remedy for fever alleviation, anti-inflammation, and diuretic effects (17). Various studies have been conducted on the diverse effects of S. hexaphylla, such as diabetes complications and osteoporosis alleviation (18, 19). Previously, we studied the effects of S. hexaphylla on benign prostate hyperplasia (BPH) by inhibiting 5α-reductase type 2 activity (20). We hypothesized that the extract of S. hexaphylla also inhibits 5α-reductase and androgen signaling in the skin.
2. Objectives
In this study, the efficacy of AGA by the extract of S. hexaphylla was examined by focusing on the androgen signal pathway.
3. Methods
3.1. Plant Material
The full name, S. hexaphylla (Thunb.) Decne, was verified by the Medicinal Plant Names Services (http://mpns.kew.org).
The samples of S. hexaphylla were three-year-old leaves and reaped in Goheung-gun, Juollanam-do, Republic of Korea. The extract of S. hexaphylla, which was obtained from Chong Kun Dang Healthcare (Seoul, Korea), as described previously and authenticated by Professor Y. H. Kim (CCBY 17002). High-performance liquid chromatography analysis for the quality control of S. hexaphylla has been described, and hederacoside D has been previously separated (20).
3.2. Cell Culture and Treatment
Human follicle dermal papilla cells (HFDPCs) (PromoCell GmbH, Heidelberg, Germany) were cultured in keratinocyte serum-free medium (PromoCell GmbH) with supplied supplement and 100 mg/mL penicillin and streptomycin (Gibco, Grand Island, NY, USA) in a 5% CO2 incubator at 37°C. Cells were used within ten passages for the experiment. The HFDPCs (1.5 × 105 cells/mL) were seeded in collagen type-I coated 96-well and 6-well plates and incubated overnight. The cells were treated with DHT (10 nM, Sigma-Aldrich, St. Louis, MO, USA) with or without finasteride (1 μM, Sigma, USA) or 50 μg/mL of S. hexaphylla extract for 24 and 72 h. Cellular morphology was observed at 200 magnification using a phase contrast microscope (Olympus, Japan).
3.3. Cell Cycle Assay
Cultured HFDPCs were seeded at a density of 2 × 105 cells in collagen type I-coated 60 mm dishes. After culturing, the medium was replaced with a starvation medium for 24 h. The cells treated with DHT or S. hexaphylla were harvested by trypsinization, washed with phosphate-buffered saline (PBS) containing 2% fetal bovine serum (Gibco), and fixed with 100% ethanol. The fixed cells were stained with FxCycle TM PI/RNase staining solution (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The cell cycle was analyzed using a flow cytometer (FACS Canto II, BC Biosciences, San Jose, CA, USA).
3.4. Experimental Animals and Androgenetic Alopecia Mouse Model
This study was performed under the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All protocols of this experiment were approved (202006A-CNU-102) by the Institutional Animal Ethics Committee of Veterinary Medicine, Chungnam National University. Male five-week-old C57BL/6 mice were supplied by Orient Bio (Seongnam, Korea) and acclimatized for 1 week under stable conditions at 22 ± 2°C, 30 % relative humidity, and a 12 h light-dark cycle before starting the experiment.
Reagents were administered as previously described, with modifications (21). The hair on the dorsal part of the mice was removed using an electric clipper to set the telogen phase. A total of 45 mice were randomly divided into five groups (n = 9/group). To induce AGA models, testosterone propionate (TP) (Tokyo Chemical Ins. Co., Tokyo, Japan) was injected subcutaneously (SC) into the dorsal region of mice in treatment groups, and control mice received SC injection of the aqueous vehicle once daily for eight weeks. Finasteride (0.1 mg/mL) or S. hexaphylla (50 mg/kg) was administered orally to most mice, except for control and AGA group mice, which were treated orally with PBS once daily for eight weeks. Two weeks after the end of the administration period, the mice were fasted overnight and were sacrificed with CO2 anesthesia. Hair growth scoring was measured weekly by the distribution of hair on the dorsal skin of mice. The skins were harvested and stored at -80°C until further analysis.
3.5. Dihydrotestosterone Level Assessment
The DHT levels in the HFDPCs and mouse skin were measured using a commercial enzyme-linked immunosorbent assay kit (MyBioSource, San Diego, CA, USA) according to the manufacturer’s instructions. Cell homogenates and serum were added with HRP-conjugate and antibody in each 96 well plates for 1 hour. After incubating, each well was aspirated and washed, and added substrate A and B solutions. After 15 minutes, a stop solution was added, and the optical density was measured using a microplate reader (INNO, LTek, Gyeonggi-do, Korea) set to 450 nm.
3.6. Histological Study
Hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) were performed as previously described (20). The 4 μm sections were deparaffinized and dehydrated. The sections were stained with hematoxylin and eosin solution for H&E or treated with 0.5 % Triton X-100 (Sigma) and 3 % H2O2 for IHC. After blocking with normal goat serum (Vector Laboratories, California, USA), the sections were incubated overnight using anti-cytokeratin 14 (CK-14) (1: 200) and anti-proliferating cell nuclear (PCNA) (1: 3000; Abcam, Cambridge, UK). The secondary antibody was attached in sections; the 3,3’-diaminobenzidine was adjusted for color development. The stained samples were pictured using a light microscope (Nikon ECLIPSE Ni-U, Tokyo, Japan) at 100X and 400X magnification and evaluated randomly in 10 areas of each slide using Image J software (Image J v1.46a; NIH, USA).
3.7. Western Blot
Western blotting was performed as previously described (20). The protein was quantified by bicinchoninic acid assay (ThermoFisher), and each sample was loaded and separated on an SDS-PAGE gels according to kDa. After samples were transferred to the membrane, the primary antibodies used were as follows: anti-androgen receptor (1: 1000) (Santa Cruz, Biotechnology, Dallas, TX, USA), anti-5α-reductase type 2 (1: 500) (Abcam), anti-AR (1: 1000) (Abcam), anti- p-GSK-3β 1: 1000) (Abcam), anti-B-cell lymphoma-associated X (Bax) (1: 1000) (Abcam), anti-B-cell lymphoma 2 (Bcl-2) (1: 1000) (Santa Cruz), anti-poly (ADP-ribose) polymerase (PARP) (1: 1000) (Cell Signaling), anti-PCNA (1: 1000) (Abcam), anti-β-catenin (1: 1000) (Abcam), anti-TGF-β1 (1: 1000) (Santa Cruz), anti-DKK-1 (1: 1000) (Abcam). Proteins were expressed with an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, Buckinghamshire, UK) and quantified using CS analyzer (ATTO, Tokyo).
3.8. TUNEL Staining
TUNEL staining was performed using the DeadEndTM Fluorometric TUNEL kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Samples were deparaffinized and pretreated with 20 μg/mL proteinase K for 15 min. After quenching in 3% hydrogen peroxide in PBS, samples were applied with equilibration buffer and working strength TdT enzyme. Samples were mounted with ProLong TM Gold Antifade Mountant with DAPI (Invitrogen). The slides were observed under a Nikon 80i microscope (Nikon).
3.9. Statistical Analysis
All experiments were conducted in double-blind conditions. The results were randomly selected and expressed as the mean ± SEM of duplicate experiments. GraphPad Prism (version 5.0; GraphPad Software, La Jolla, CA, USA) was used for analysis and interpretation. Significant differences were analyzed using the Mann-Whitney U test for non-parametric data between two groups and the post-hoc Tukey test for multiple groups when relevant (Sigmaplot 12.0). Statistical significance was set at P < 0.05.
4. Results and Discussion
This study investigated the effectiveness of S. hexaphylla in AGA in HFDPCs and a TP-induced AGA mouse model. Previously, we found that the extract of S. hexaphylla plays the role of 5α-reductase inhibitor in the prostate by inducing apoptosis (20). The main constituents of S. hexaphylla are hederacosdie D, chlorogenic acid, and luteolin, which are associated with anti-inflammatory and antioxidant effects (22, 23). Among them, hederacoisde D, which is a bioactive saponin, was isolated from the extract of S. hexaphylla and studied to improve BPH (20, 24). We hypothesized that the extract of S. hexaphylla also inhibits 5α-reductase and androgen signaling in the skin to improve AGA. Interestingly, S. hexaphylla also acted as a 5α-reductase inhibitor in the skin, but the results were the opposite.
4.1. Extract of Stauntonia hexaphylla Inhibiting 5α-reductase and Androgen Receptor Resulting in Reducing Apoptosis and Inducing Cell Proliferation in Human Follicle Dermal Papilla Cells
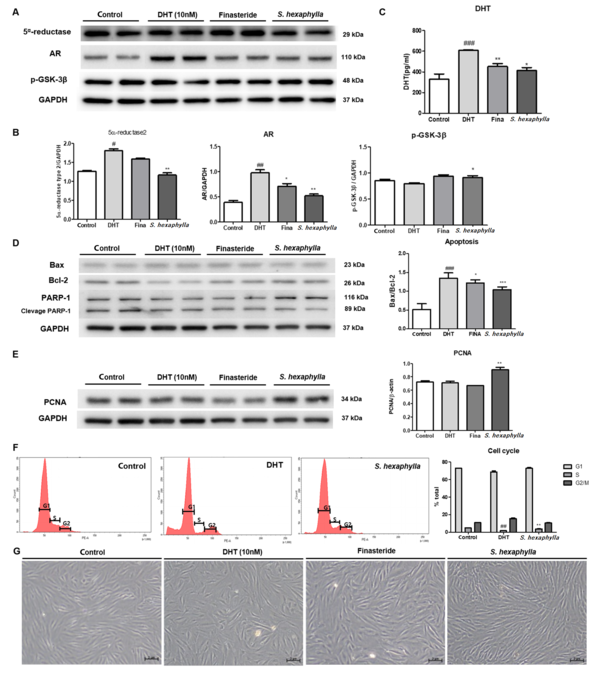
The effects of S. hexaphylla on androgen signaling in HFDPCs were evaluated using western blotting. In HFDPCs, DHT treatment significantly (P < 0.01) upregulated the expression of AR signaling factors, such as 5α-reductase, DHT, and AR. However, S. hexaphylla treatment significantly (P < 0.005) reduced the expression of 5α-reductase, DHT, and AR compared to DHT treatment. In addition, S. hexaphylla treatment increased the expression of p-GSK-3β compared to DHT treatment due to AR expression (Figure 1A - C). As shown in Figure 1D, the ratio of Bax/Bcl-2 was significantly (P < 0.001) increased twofold following DHT treatment in HFDPCs compared to the controls. However, treatment with S. hexaphylla significantly (P < 0.001) reduced the expression of Bax/Bcl-2 compared to DHT treatment. Under the S. hexaphylla treatment, the expression of Bax did not appear to change significantly, but the expression of BCL-2 was increased dominating the Bax/Bcl-2 ratio. The extract of S. hexaphylla suppressed the expression of 5α-reductase and AR by inhibiting apoptosis.
The effects of Stauntonia hexaphylla extract on androgen signaling, apoptosis, and proliferation in human follicular dermal papilla cells (HFDPCs). Human follicular dermal papilla cells were starved for 24 h, and dihydrotestosterone (DHT) (10 nM) was treated with or without finasteride (10 nΜ) and S. hexaphylla extract (50 μM) co-treatment for 72 h. A, 5α-reductase, androgen receptor, and p-glycogen synthase kinase-3β (GSK3β); B, Relative quantitative densitometry evolution of western blot data; C, Level of dihydrotestosterone; D, The expression of Bax, Bcl-2, and poly (ADP-ribose) polymerase (PARP)-1 and relative quantitative densitometry evolution of western blot data; E, Expression of proliferating cell nuclear antigen (PCNA); F, Cell cycle; G, Cell morphology 200X. Values are expressed as mean ± SEM. *** P < 0.001, ** P < 0.01 and * P < 0.05, significant differences compared with DHT-treated cells. ### P < 0.001, significant differences compared with control cells.
In addition, S. hexaphylla not only affects apoptosis but affects the cell cycle. As shown in Figure 1E, PCNA expression was increased in S. hexaphylla-treated HFDPCs (Figure 1E). Based on the proliferation results, we examined the effect of S. hexaphylla on the cell cycle of HFDPCs. The G0/G1 phase remained unchanged after DHT treatment. Interestingly, the S phase was significantly (P < 0.001) increased, and the G2 phase was reduced by S. hexaphylla treatment compared to DHT treatment (Figure 1F). Dihydrotestosterone treatment showed increased Sub-G1 and G2/M phases and reduced S phase, meaning apoptotic cells increased and G2 arrest (25). Conversely, S. hexpahylla-treated cells exhibited increased DNA replication, representing an increase in the S phase. Furthermore, the morphology of cells is shown in Figure 1G. In the HFDPCs treated with DHT, the shape of cells was shortened, whereas the cell was thinner and longer in S. hexaphylla-treated HFDPCs. This result suggests that S. hexaphylla regulates hair growth cycle factors by increasing proliferation.
4.2. Extract of Stauntonia hexaphylla for Hair Growth in Testosterone Propionate-induced Androgenetic Alopecia Mice Model
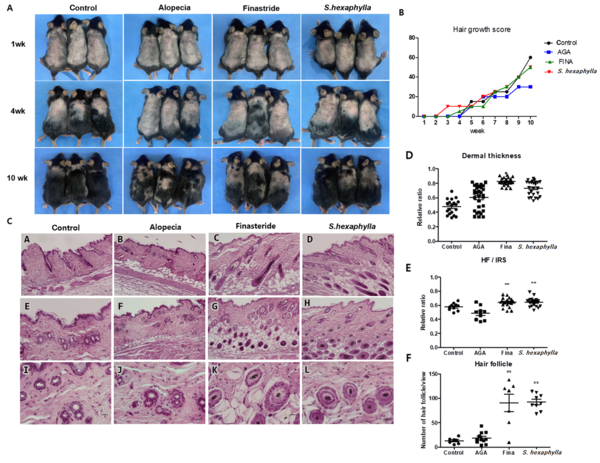
Based on the results of androgen signaling expression and cell cycle assay in HFDPCs, we confirmed that the extract of S. hexaphylla has the potential to reduce AGA and evaluated the hair cycle time in a TP-induced AGA mouse model for 10 weeks. Hair growth and hair growth scores are shown in Figure 2A and B. At four weeks, the hair started to grow in all groups except the alopecia groups, with less than 10% growth in the AGA group. In addition, hair quantities were higher in the S. hexaphylla group compared to the AGA group. Finally, at 10 weeks, the hair growth scores of the finasteride and S. hexaphylla groups were more than 50, which is higher than that of the AGA group. Figure 2C shows the histological views of the longitudinal and transverse sections.
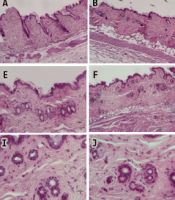
Analysis of morphological and histological changes caused by Stauntonia hexaphylla extract in the testosterone propionate (TP)-induced androgenic alopecia (AGA) mouse model. A, Comparison of back skin colors and hair growth in TP-induced C57BL/6 mice; B, Hair growth score; C, Comparison of histological images of hair follicles (longitudinal view, A - D (100X) transverse view, E - H (100X), I - L (400X); D, the dermis, S; subcutaneous); D, Dermal thickness; E, Quantification of cross-sectional diameters of the hair shaft/inner root sheath (IRS); F, Follicular numbers were assessed. Values are expressed as mean ± standard deviation. ** P < 0.01 and * P < 0.05, significant differences compared with the AGA group.
The anagen I-II phases were observed in the control groups. However, the telogen phase was observed in the AGA group, showing miniaturization of HFs by repeated shortening of the anagen phase and prematuring the catagen phase. Conversely, HFs were observed in the dermis and hypodermis representing the anagen IIIc-VI phases diversely in finasteride and S. hexaphylla groups, indicating a prolonged anagen phase and delayed induction of the catagen phase compared to the AGA group (2). The dermal thickness (P < 0.05) and the number of HFs (P < 0.01) were significantly increased in the finasteride and S. hexaphylla groups compared to the AGA group. In addition, the cross-sectional diameters of the hair shaft/inner root sheath (IRS) were lower in the AGA group than in the control group. However, S. hexaphylla significantly increased the cross-sectional diameter of the hair shaft/IRS (P < 0.01). Comprehensively, the indicators, dermal thickness, hair bulb diameter, and number of HFs indicated the presence of the anagen phase in finasteride and S. hexaphylla groups, which prolonged the anagen phase compared to the AGA group.
4.3. Extract of Stauntonia hexaphylla Inhibiting 5α-reductase and Androgen Signaling resulting in Upregulation of p-Glycogen Synthase Kinase-3β and β-catenin in Testosterone Propionate-induced Androgenetic Alopecia Mice Model
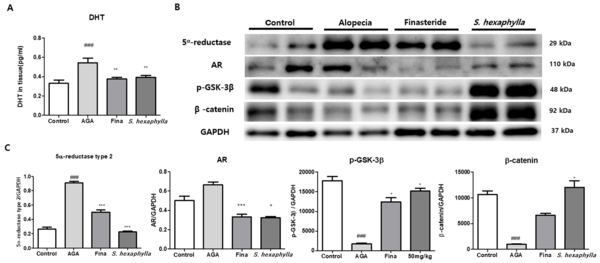
Figure 3 shows that the expression of 5α-reductase was significantly (P < 0.001) increased in the AGA groups but was markedly (P < 0.001) reduced in the finasteride and S. hexaphylla groups. Corresponding to the results of 5α-reductase expression, the concentration of DHT in the tissues is shown in Figure 3A. The DHT level in the AGA group was significantly (P < 0.001) higher than that in the control group. However, S. hexaphylla treatment decreased the DHT levels to the same extent as finasteride treatment. Furthermore, AR expression was increased in the AGA group and decreased in the finasteride and S. hexaphylla groups. In contrast to the results of AR expression, the expression levels of p-GSK-3β and β-catenin were significantly (P < 0.001) decreased in the AGA groups and significantly (P < 0.001) increased in the S. hexaphylla group.
The effects of Stauntonia hexaphylla extract on androgen signaling in the testosterone propionate (TP)-induced androgenic alopecia (AGA) mouse model. A, Dihydrotestosterone levels in the skin were determined using a commercial assay kit; B, 5α-reductase, androgen receptor, p-glycogen synthase kinase-3β (GSK3β), and β-catenin were examined; C, Relative quantitative densitometry evolution of western blot data. Values are expressed as mean ± standard deviation. ### P < 0.01, significant differences compared with control cells. *** P < 0.001 and * P < 0.05, significant differences compared with the AGA group.
Dihydrotestosterone and AR signaling activate GSK-3β, inhibiting the Wnt pathway and insulin-like growth factor-1, a hair growth factor (8, 26). Dihydrotestosterone, a key inducer of AGA, forms DHT-AR complex leading to the dephosphorylation of GSK-3β. This mechanism degrades the β-catenin and downregulates Wnt/β-catenin signaling, which is mainly involved in the hair growth pathway (8, 27). In our study, the 5α-reductase type 2 reduced markedly in S. hexaphylla groups compared to that in the AGA group, resulting in a low DHT concentration in the skin. In addition, AR expression was significantly reduced in S. hexaphylla groups, leading to increased GSK-3β phosphorylation and upregulated β-catenin. By phosphorylating GSK-3β resulting from reduced AR-DHT complex levels, GSK-3β activation was inhibited and β-catenin induced.
4.4. Extract of Stauntonia hexaphylla Downregulating Apoptosis and Upregulating Keratinocyte Proliferation in Testosterone Propionate-induced Androgenetic Alopecia Mice Model
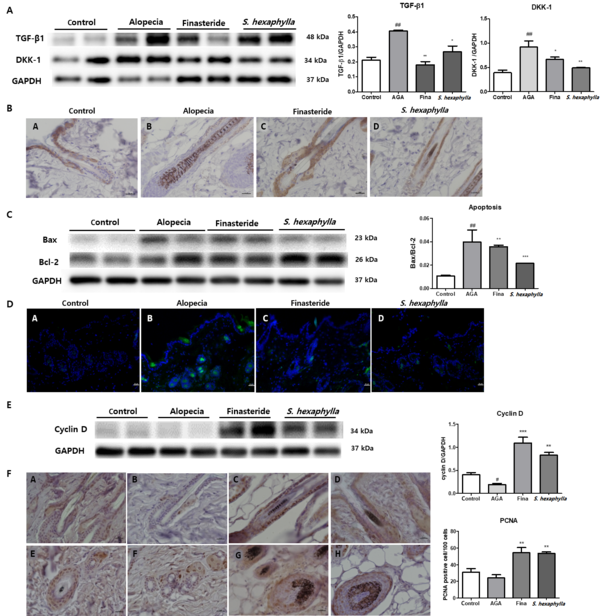
Figure 4A shows the expression levels of TGF-β1 and DKK-1. Transforming growth factor beta-1 and DKK-1 expression was increased in the AGA groups but was reduced in the S. hexaphylla group compared to the AGA group. Paracrine factors such as TGF-β1 and DKK-1, involved in outer root sheath keratinocytes (KCs), were upregulated in the AGA group compared to the control group. Androgens, especially DHT, induce IL-6, TGF-β1, and DKK-1 (28-30). Transforming growth factor beta-1 suppresses the growth of epithelial KCs and induces the catagen phase (31). Dickkopf-a is a natural Wnt inhibitor that induces KC apoptosis in the prematuration catagen phase (29, 32). These factors induced apoptosis and further progressed hair loss. Our results revealed that TGF-β1 and DKK-1 were downregulated in S. hexaphylla groups inducing KC proliferation and suppressing apoptosis. Immunohistochemical analysis revealed that CK-14 was expressed in the outer root sheath. The CK-14-positive cells were increased in finasteride and S. hexaphylla groups compared to those in the AGA group (Figure 4B).
The effects of Stauntonia hexaphylla extract on paracrine factors, apoptosis, and proliferation in the TP-induced androgenic alopecia (AGA) mouse model. A, transforming growth factor beta-1 (TGF-β1), dickkopf-a (DKK-1), and relative quantitative densitometry evolution of western blot data; B, Immunohistochemistry of cytokeratin 14 (CK-14) in the outer root sheath, 400X (A, control; B, alopecia; C, finasteride; D, S. hexaphylla 50 mg/kg); C, Bax, Bcl-2, and the Bax/Bcl-2 ratio indicating apoptosis; D, TUNEL staining 100X (A, control; B, alopecia; C, finasteride; D, S. hexaphylla 50 mg/kg); E, Cyclin D and relative quantitative densitometry evolution of western blot data; F, Immunohistochemistry of proliferating cell nuclear antigen (PCNA) and the number of positively stained nuclei in the prostate epithelial cells. Longitudinal view (A - D, 400X); transverse view (E - H 400X) (A and E, control; B and F, alopecia; C and G, finasteride; D and H, S. hexaphylla 50 mg/kg). Values are expressed as mean ± standard deviation. ## P < 0.01 indicates significant differences compared with control cells. *** P < 0.001, ** P < 0.01 and * P < 0.05 indicate significant differences compared with the AGA group.
In a while, Bax expression was increased in the AGA group, it was reduced in the S. hexaphylla group compared to that in the AGA group. In addition, Bcl-2 expression was increased in S. hexaphylla group. The ratio of Bax/Bcl-2, which is related to apoptosis, was significantly (P < 0.01) increased in the AGA group and markedly reduced in the S. hexaphylla groups, indicating reduced apoptosis (Figure 4C). TUNEL staining also showed that the number of positive cells was increased in the AGA group and decreased in the S. hexaphylla group, which supports the aforementioned fact (Figure 4D). Taken together, S. hexaphllya inhibited androgen signaling, resulting in KC proliferation and reduction of apoptosis by reducing paracrine factors.
β-catenin can translocate into the nucleus, and the cyclin D1 gene is a target for the β-catenin (33). In the cell cycle, cyclin D determines whether cell division progresses in cells that have completed the M phase from G0 to the G1 phase (34). Our finding is that the expression of cyclin D was significantly (P < 0.005) increased in S. hexaphylla groups while decreasing in the AGA group. These results also appear to be due to induced β-catenin which upregulated cyclin D expression in the skin as a result of inhibiting androgen signaling in S. hexaphylla groups. Moreover, the number of PCNA-positive cells, which were stained in the outer sheath of HFs, were significantly increased, indicating S. hexaphylla caused cell growth in the skin. These results suggest that S. hexaphylla induces cell cycle and increased cell growth, which appears to be due to the blockade of androgen signaling.
5. Conclusions
In conclusion, this study demonstrated that S. hexaphylla extract was a 5α-reductase inhibitor and downregulated androgen signal in AGA, resulting in the phosphorylation of GSK-3β and reduction of paracrine factors, TGF-β1 and DKK-1. These results showed inhibition of apoptosis and catagen prematuration in response to S. hexaphylla treatment. In addition, S. hexaphylla induces KC proliferation by inducing β-catenin and upregulating PCNA and cyclin D. We suggest that S. hexaphylla has potential effects in alleviating AGA by inhibiting 5α-reductase and androgen signaling.