1. Background
Postoperative cognitive dysfunction (POCD) is a serious complication that usually occurs after anesthesia and major surgery in elderly patients. It is manifested as cognitive dysfunction that affects learning and memory, which might increase complications and mortality (1, 2). Studies have shown that the anesthetics, such as sevoflurane, might cause neuronal apoptosis in experimental animals and elderly patients with Alzheimer’s disease, which highlighted the role played by neurotoxicity in the patients (3-5). The quality of the patient’s life following surgery can be improved by understanding the pathogenesis and mechanism of POCD induced by inhaling the sevoflurane anesthetic drug and then identifying the effective targets to prevent neurotoxicity and cognitive dysfunction.
Autophagy is a defensive behavior of the body in response to local hypoxia or anesthetic inhalation. However, increased hippocampal autophagy could lead to persistent brain damage and cognitive dysfunction (6, 7). Koike et al. developed a mouse model with acute ischemic brain injuries by ligating the left common carotid artery (8). They applied the immunofluorescence technique to detect the presence of several light chain-3 granules in the left hippocampal pyramidal cells after 8 hours of ischemia, which indicated that autophagic activity exists in the damaged hippocampal neurons (8).
The hippocampus is the primary site of action for inhaled anesthetics and is closely related to cognitive function. The destruction of the hippocampal structure can lead to various central nervous system diseases. The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway was overactivated in various types of cancer. This pathway also regulates many cellular mechanisms, such as cell growth, metabolism, survival proliferation, angiogenesis, and metastasis (9-11). Recently, it was noted that this pathway was closely associated with autophagy (12). An inactivated mTOR signaling pathway enhances autophagy and plays a crucial role in POCD onset (13). The PI3K/Akt/mTOR autophagy pathway was also significantly attenuated in the aged rat model of POCD induced by the inhalation anesthetics and is associated with oxidative stress and neuroinflammation (14).
Melatonin, primarily synthesized and secreted by the mammalian and human pineal glands, plays a significant role in various physiological functions, including endocrine, anti-aging, anti-inflammatory, and anti-oxidative stress regulation and apoptosis reduction (15, 16). Many recent studies showed that melatonin regulates reperfusion injuries via the mechanism of inhibiting autophagy-related protein expression by activating the PI3K/Akt/mTOR signaling pathway (17). In addition, a decreased serum melatonin level was determined to be an independent risk factor that led to postoperative delirium in elderly patients (18). A recent meta-analysis showed that exogenous melatonin supplementation could improve perioperative cognitive function in elderly patients; however, its specific mechanism and targets are yet to be determined (19).
2. Objectives
The current study hypothesized that melatonin plays a neuroprotective role by regulating the PI3K/Akt/mTOR signaling pathway in the sevoflurane-induced POCD model, which could provide novel therapeutic strategies for clinical treatment.
3. Methods
3.1. Chemicals and Antibodies
Melatonin (pure: 99.73%), LY294002 (pure: 99.92%), and rapamycin (pure: 99.52%) were purchased from MedChemExpress (New Jersey, USA). Anti-phosphorylated (p)-Akt, anti-Akt, anti-p-mTOR, anti-mTOR, anti-coiled-coil, myosin-like BCL2 interacting protein (Beclin 1), anti-microtubule-associated proteins light chain-3 II (LC3-II), anti-B cell lymphoma 2 (Bcl-2), anti-Bcl2 associated X-protein (Bax), anti-cleaved caspase-3, anti-ionized calcium-binding adapter molecule 1 (IBA-1), anti-interleukin (IL)-1β, anti-IL-6, anti-inducible nitric oxide synthase (iNOS), anti-β-actin, anti-glyceraldehyde 3 phosphate dehydrogenase, and horseradish peroxidase (HRP)-conjugated goat anti-rabbit antibodies were obtained from Affinity Biosciences (New Jersey, USA). Anti-p-PI3K and anti-PI3K antibodies were purchased from Cell Signaling Technology (Boston, USA).
3.2. Animals Used in the Study
C57BL/6J mice (n = 94; aged 20 - 22 months) were procured from Shanghai Legend Biotechnology Co., Ltd. (China). At the beginning of the experiment, the mice were randomly assigned to six mice per cage. The mice were reared in standard cages at 60% indoor relative humidity and temperatures of 24 - 26°C under 12 hours of light and dark cycles (each), with unrestricted access to food and water. The animals were left for at least a week to adjust to these conditions.
All experimental procedures were approved by the Animal Use and Care Committee for Research and Education of Jiaxing University (Jiaxing, China) and conducted in accordance with the guidelines published in the European Communities Council Directive of November 24, 1986 (86/609/EEC) (ethics approval reference no.: JUMC2022-085). This study only used male mice. The animal models were kept in a plastic-enclosed anesthetic chamber and were exposed to only oxygen (O2) (control group, C) or 2% sevoflurane and O2 (sevoflurane group, S) for 6 hours. The concentration of intraluminal sevoflurane was monitored using an automated anesthetic gas monitor every 2 minutes.
Following anesthesia, the animals were housed in their earlier living space. In the first experiment, the mice were randomly categorized into four different groups, namely: (1) C; (2) C + melatonin (10 mg/kg) (C + M); (3) sevoflurane group (S); and (4) S + melatonin (10 mg/kg) group (S + M). In the second experiment, the animals were randomly categorized into four groups, namely: (1) S; (2) S + M (10 mg/kg); (3) S + M (10 mg/kg) + PI3K/Akt inhibitor LY294002 (30 mg/kg) group (S + M + L); and (4) S + M (10 mg/kg) + mTOR inhibitor rapamycin (10 mg/kg) group (S + M + R). All drugs were administered intraperitoneally immediately after sevoflurane or O2 treatment. Behavioral testing was performed 24 hours following sevoflurane anesthesia.
3.3. Morris Water Maze Test
Based on a previous study (20), the mice’s long-term spatial learning and memory impairment, anesthetized with sevoflurane, were determined as per the Morris water maze test. The mice were trained over 5 consecutive days for 60 seconds, wherein they explored the immersive platform four consecutive times in one day. The camera system automatically recorded the swimming path of these animals and analyzed them with the help of corresponding software. The mice had a maximum search duration of 60 seconds, and a successful search was defined as one in which the mice stayed on the hidden platform for 3 seconds. Afterward, the mice were allowed to remain on the platform for 15 seconds to explore their surroundings. If the mice could not find the secret platform in the 60-second interval, they were led to it and permitted 20 seconds to stay on this platform. This was carried out to ensure that all mice experienced the same training intervals. The mice were mounted in the opposite quadrant without the platform during the probing test. The swimming speed, escape latency, target quadrant swimming time and distance, and cross-platform times of the mice were recorded over 60 seconds to assess their spatial learning and memory abilities.
3.4. Open Field Test
For the open field test, the mice were housed in an open square plywood box and painted with flat black enamel paint (overall measurements of 72 cm (length), 30 cm (height), and 72 cm (breadth)) (21). Artificial light and darkness conditions were used to simulate the day and night environment. A camera was mounted on the box to track the animal’s average trip distance within the box, their bout time, travel path, and time spent in the central region for 5 minutes. EthoVision XT software (version: 17.0; Noldus Information Technology, Wageningen, Netherlands) was used to process the images.
3.5. Tissue Preparation
With reference to previous studies (22, 23), following behavioral testing, the mice were euthanized using pentobarbital (1.5% sodium pentobarbital, 0.1 mL/20 g). The animals were then dissected, and their brain tissues were extracted and placed on ice. After carefully peeling off the skull, the brain tissue was completely removed, followed by a rapid removal of the hippocampus within 3 minutes. The dissected hippocampal tissue was sealed in a cryovial and frozen in liquid nitrogen to extract the total protein samples. The hippocampus tissue was fixed using 4% (w/v) formaldehyde, embedded into a paraffin block, and sliced to obtain coronal sections (4 μm). These sections were further stained using the hematoxylin and eosin (H&E) staining technique.
3.6. Western Blotting Technique
According to a previous study (24), the hippocampal tissue samples were sectioned into small pieces, weighed, and placed into Eppendorf tubes. Then, radioimmunoprecipitation assay (RIPA) lysis buffer, comprising phenylmethylsulfonyl fluoride (a protease inhibitor), was added to the Eppendorf tubes (containing RIPA lysis buffer (200 μL) per 10 mg of a tissue sample), following which the samples were placed in an ice bath for 30 minutes. Thereafter, the tissue samples were ground four times (30 seconds each) using an automatic sample grinder at 60 Hz. These samples were then centrifuged at a rate of 12000 rpm (4°C) for 5 minutes.
The cell-free supernatant that was obtained was used as the total protein sample. The concentration of the total protein was determined using the bicinchoninic acid assay. Then, the loading buffer was added to the protein sample (in a 1: 4 ratio), and all samples were boiled at 100°C for 5 minutes. Afterward, these samples were loaded onto the sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4 - 20%) and electrophoresed to separate the different proteins in the samples. These gels were then transferred to polyvinylidene difluoride (PVDF) membranes to transfer the proteins onto the membranes under standard blotting conditions. These PVDF membranes were blocked using 10% (v/v) skimmed milk for 60 minutes, washed with the tris-buffered saline with 0.1% Tween® 20 solution, and incubated with a primary antibody at 4°C overnight (all 1: 1000). Then, PVDF membranes were incubated using the secondary goat anti-rabbit antibody, conjugated to HRP. Based on the manufacturer’s instructions, electrochemiluminescence reagents were used to detect the bands. Image-Pro Plus software (version: 7.0) was used to conduct densitometric analysis on Western blotting films.
3.7. Histochemical Staining
The hippocampal tissue samples were collected and fixed using a 4% neutral formalin solution and embedded into the paraffin block. Then, thin sections were sliced. The tissue sections (4 μm) were deparaffinized in xylene, then rehydrated by reducing the ethanol content, washed using the phosphate-buffered saline solution, and stained using H&E staining (25). Following the staining procedure, the sections were further dehydrated by adding more ethanol and xylene. The Fluorescence microscopy technique (Olympus, Japan) was used to examine the neuronal damage occurring in the hippocampus.
3.8. Statistical Analysis
The GraphPad Prism software (version 6.0, USA) was employed to statistically analyze the data, which was presented as mean ± standard error of the mean. The cutoff value for statistical significance was P < 0.05.
4. Results
4.1. Attenuative Effects of Melatonin on Sevoflurane-induced Cognitive Impairment in Aged Mice
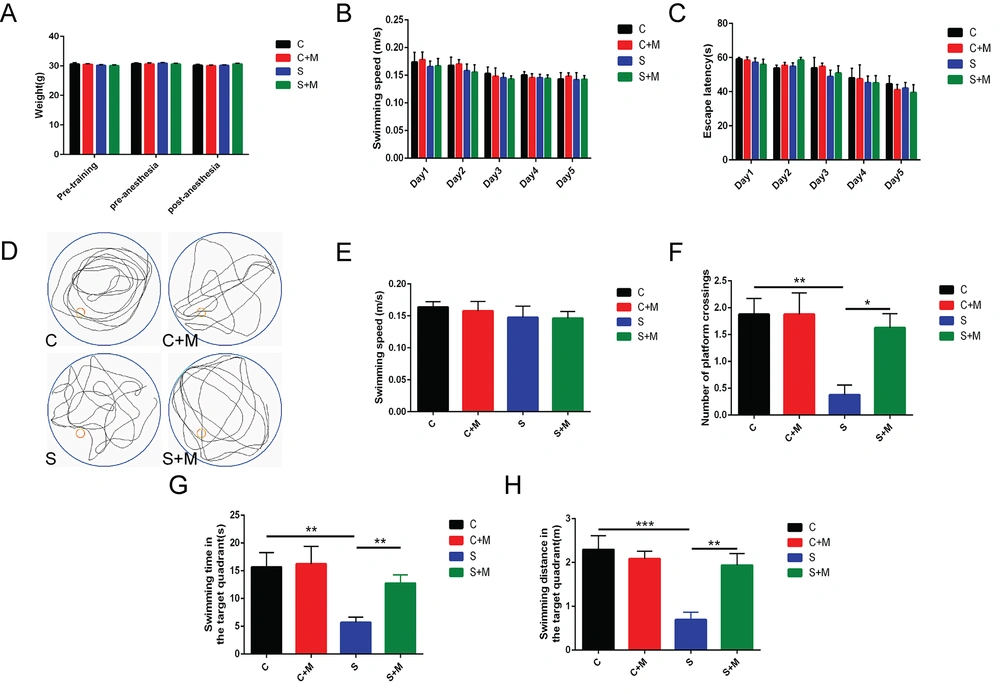
In this study, the Morris water maze test was carried out on the aged mice to examine the effect of melatonin on the cognitive functioning in the brain of aged mice impacted by sevoflurane anesthesia. As shown in Figure 1A, no significant difference was noted in the body weights of the animals across groups before training, before anesthesia, and after anesthesia. Furthermore, the findings did not reveal a significant statistical difference in the escape latency and swimming speed of mice in each group during the 5 consecutive days of water maze training (Figure 1B and C). Twenty-four hours after sevoflurane anesthesia, groups C and C + M showed clear swimming paths, indicating that their recognition abilities were unaffected (Figure 1D). Additionally, no statistically significant variation was noted in the swimming speed of the mice in each group (P > 0.05 vs. group C; P > 0.05 vs. group S; n = 8, one-way analysis of variance (ANOVA), Figure 1E).
Effect of melatonin on cognitive impairment induced by sevoflurane; A, body weight of mice in each group; B, swimming speed; C, escape latency; D, swimming path; E, swimming speed; F, times of platform crossing; G, target quadrant swimming time; H, target quadrant swimming distance; data showing mean ± standard error of mean (SEM); * P < 0.05, ** P < 0.01, *** P < 0.001
The number of times the animals crossed the platform, the duration of their swimming exercise in a target quadrant, and the swimming distance in this target quadrant were observed to be significantly reduced in group S than in group C; nevertheless, the values increased in the S + M group in comparison to S group (** P < 0.01, *** P < 0.001 vs. group C; * P < 0.05, ** P < 0.01 vs. group S; n = 8, one-way ANOVA, Figure 1F - H). The aforementioned findings suggested that melatonin could reverse the inhibitory effect of sevoflurane exposure on memory and learning neurological dysfunctions in aged mice.
4.2. Rescue of Down-regulation of the Phosphatidylinositol 3-Kinase/Protein Kinase B/ Mammalian Target of Rapamycin Signaling Pathway by Melatonin in the Sevoflurane-anesthetized Aged Mice
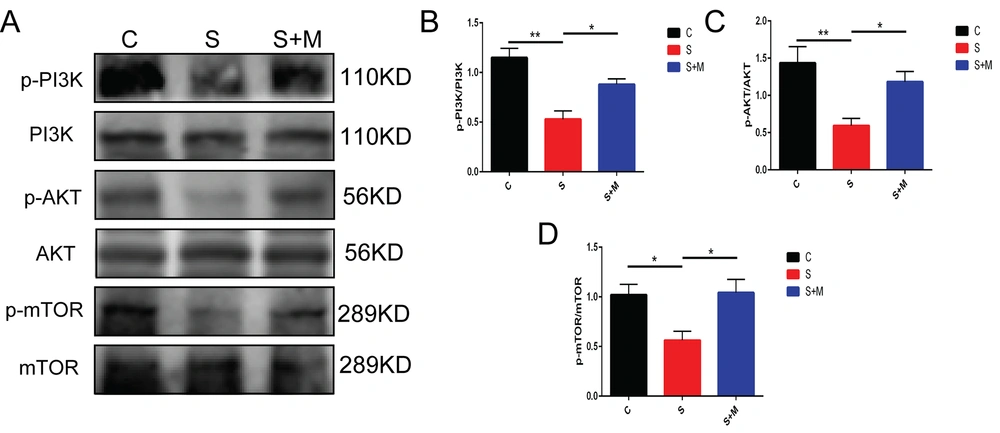
Previous studies have highlighted the role of the hippocampal PI3K/Akt/mTOR signaling pathway in cognitive dysfunction. Therefore, this study investigated the effect of melatonin on the hippocampal PI3K/Akt/mTOR signaling pathway in aged mice. The Western blotting results showed that, compared to group C, the S-group mice showed a lower protein expression level of p-PI3K, p-Akt, and p-mTOR in the hippocampus region. The findings also showed that melatonin could significantly reverse this effect (* P < 0.05, ** P < 0.01 vs. group C; * P < 0.05 vs. group S; n = 8, one-way ANOVA, Figure 2A - D). The aforementioned findings suggested that melatonin could modulate the hippocampal PI3K/Akt/mTOR signaling pathway in sevoflurane-anesthetized elderly mice.
Effects of melatonin on the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathways; A - D, western blotting applied to measure the expression of phosphorylated (p)-PI3K, PI3K, p-Akt, Akt, p-mTOR, and mTOR; data showing mean ± standard error of mean; * P < 0.05, ** P < 0.01
4.3. Amelioratory Effects of Melatonin on Cognitive Function in Aged Mice by Regulating the Phosphatidylinositol 3-Kinase/Protein Kinase B/ Mammalian Target of Rapamycin Signaling Pathway
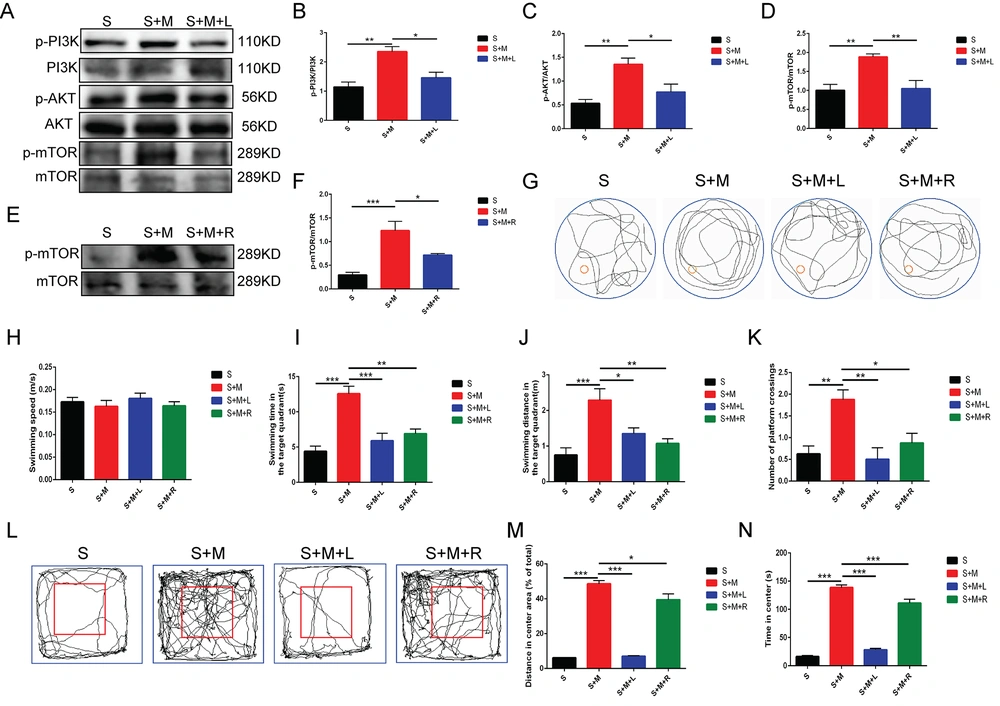
To further investigate the specific mechanism of melatonin, LY294002, an inhibitor of the PI3K/Akt signaling pathway, and rapamycin, an mTOR inhibitor, were combined to treat the aged mice following sevoflurane anesthesia. The Western blotting results confirmed that, in comparison to the S + M group, the S + M + L and S + M + R groups could significantly inhibit the p-PI3K, p-Akt, and p-mTOR protein expression in the hippocampus (** P < 0.01, *** P < 0.001 vs. group S; * P < 0.05, ** P < 0.01 vs. group S + M; n = 4, one-way ANOVA, Figure 3A - F). The subsequent water maze results revealed that although the swimming speed of the animals did not significantly differ across groups, the mice in the S + M group showed more platform crossings and an increased swimming distance and time in the target quadrant, compared to the S-group mice. This effect could be reversed after combining LY294002 and rapamycin, respectively (** P < 0.01, *** P < 0.001 vs. group S; * P < 0.05, ** P < 0.01, *** P < 0.001 vs. S + M group; n = 8, one-way ANOVA, Figure 3G - K). The open field results revealed that sevoflurane exposure significantly reduced the time and distance for which the mice stayed in the center; however, melatonin reversed this event. This effect of melatonin was further reversed by PI3K/Akt/mTOR inhibitors (*** P < 0.001 vs. group S; * P < 0.05, *** P < 0.001 vs. group S + M; n = 8, one-way ANOVA, Figure 3L - N). The aforementioned findings imply that melatonin could enhance memory and learning abilities in elderly animals after modifying the PI3K/Akt/mTOR signaling pathway.
Amelioratory effect of melatonin on cognitive function by regulating phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) signaling pathway; A - F, the protein expressions of phosphorylated (p)-PI3K, PI3K, p-Akt, Akt, p-mTOR, and mTOR detected by western blotting; G, swimming path; H, swimming speed; I, target quadrant swimming time; J, target quadrant swimming distance; K, times of platform crossing; L, representative traces of locomotor activity in the open field; M, distance travelled in the central area in the open field; N, time spent in the central area in the open field; data showing mean ± standard error of mean; * P < 0.05, ** P < 0.01, *** P < 0.001
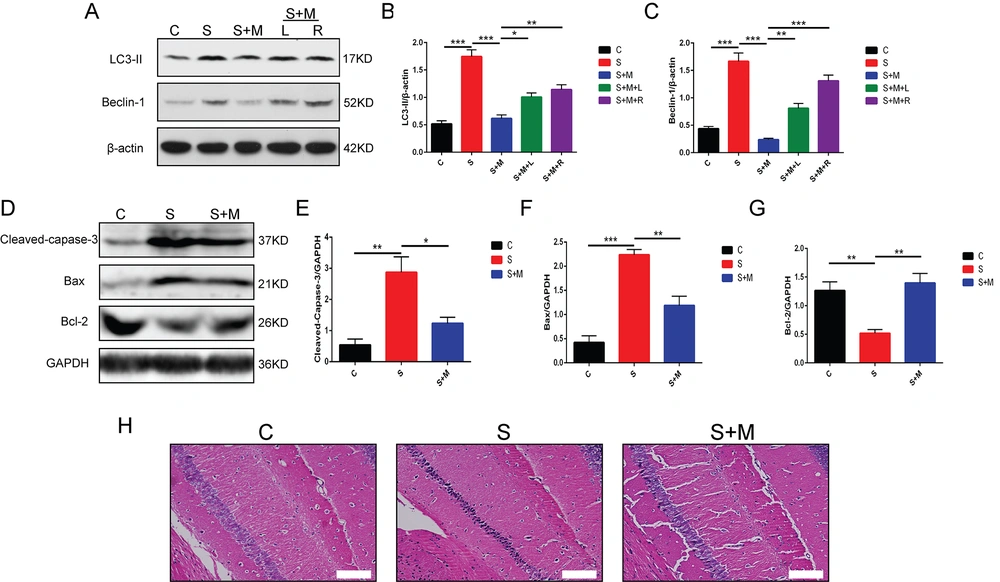
4.4. Inhibitory Effects of Melatonin on Sevoflurane-induced Autophagy-related Protein Expression and Neuronal Apoptosis in the Hippocampus Region of Aged Mice
Recent studies showed that an inactivated mTOR signaling pathway leading to enhanced autophagy plays a crucial role in cognitive dysfunction progression. Therefore, in this study, a few experiments were conducted to determine whether melatonin affects the autophagy-related protein expression in the hippocampus region of elderly mice. The Western blotting results revealed that autophagy-related LC3-II and Beclin 1 protein expression levels in the hippocampus of the S-group mice were increased significantly than in the C-group mice. This finding indicated that 2% sevoflurane exposure induced the activation of the autophagic pathways in the hippocampus of elderly mice, which could be effectively reversed by melatonin. The combined PI3K/Akt/mTOR inhibitor reversed the inhibitory impact of melatonin (*** P < 0.001 vs. group C; *** P < 0.001 vs. group S; * P < 0.05, ** P < 0.01, *** P < 0.001 vs. group S + M; n = 8, one-way ANOVA, Figure 4A - C). The aforementioned findings of this study indicated that melatonin regulated autophagy-related protein expression in the hippocampus via regulating the PI3K/Akt/mTOR signaling pathway.
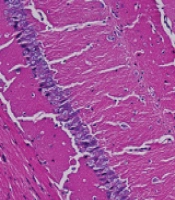
Effects of melatonin on the autophagy-related protein expression and neuronal apoptosis; A - C, the protein expressions of microtubule-associated proteins light chain 3 II (LC3-II) and coiled-coil, myosin-like BCL2 interacting protein (Beclin 1) detected by western blotting; D - G, the protein expressions of cleaved-caspase-3, Bcl2-associated X-protein (Bax), and B cell lymphoma 2 (Bcl-2) detected by western blotting; H, hematoxylin and eosin (H&E) staining of mouse hippocampal tissue; scale bars: 50 μm; data showing mean ± standard error of mean; * P < 0.05, ** P < 0.01, *** P < 0.001
Furthermore, the hippocampal neuronal apoptosis pathway was also investigated to further explore the possible mechanism used by melatonin. The Western blotting results confirmed that, compared to group C, the Bax and cleaved caspase-3 proteins were overexpressed in the hippocampus region of the S-group mice; nevertheless, the Bcl-2 protein was under-expressed. This phenomenon was noted to be reversed by melatonin (** P < 0.01, *** P < 0.001 vs. group C; * P < 0.05, ** P < 0.01 vs. group S; n = 4, one-way ANOVA, Figure 4D - G).
Following H&E staining, it was found that the hippocampus neurons in the animals in group C were neatly aligned, with a complete cellular structure, along with regular and large nuclei. The S-group mice showed chromatin condensation and nuclear pyknosis, which demonstrated that the neurons were impaired and the rate of apoptosis increased. In comparison to the S-group mice, the S + M group mice showed neatly-aligned cells with a complete cell structure (Figure 4H). The aforementioned findings suggested that melatonin could effectively prevent neuronal death in the hippocampus of elderly mice anesthetized by sevoflurane.
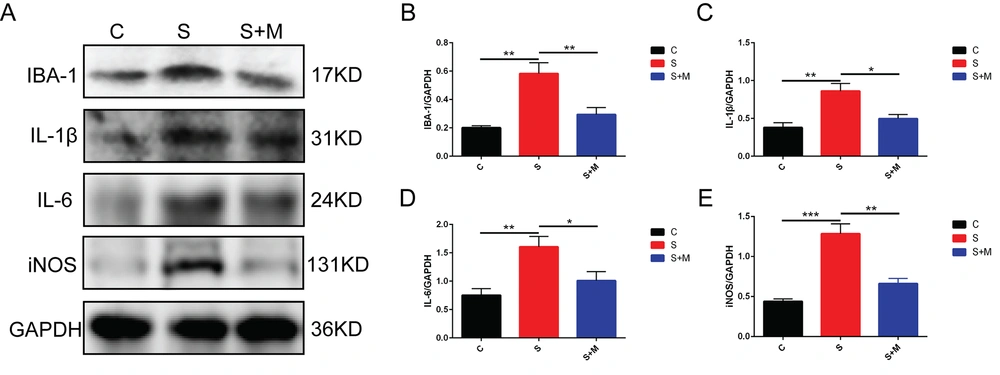
4.5. Decreasing Effect of Melatonin on Sevoflurane-induced Hippocampal Glial Cell Activation and Inflammatory Factor Levels in Aged Mice
Neuroinflammation is a major factor that leads to cognitive dysfunction. Previous studies have reported that melatonin inhibits glial cell activation. Therefore, the effect of melatonin was determined on neuroinflammation in the hippocampal region in mice. The Western blotting results revealed that the expression levels of IBA-1, IL-6, IL-1β, and iNOS proteins in the hippocampus regions in the S-group mice were elevated significantly than in the C-group mice. However, the IBA-1, IL-6, IL-1β, and iNOS expression levels in the hippocampus of the S + M group mice were significantly lowered, compared to the S-group mice (** P < 0.01, *** P < 0.001 vs. group C; * P < 0.05, ** P < 0.01 vs. group S; n = 4, one-way ANOVA, Figure 5A - E). The aforementioned findings indicated that sevoflurane could induce neuroinflammation in the hippocampal region, and melatonin exerts anti-inflammatory effects on the brain tissues of the elderly mice anesthetized using sevoflurane.
Effects of melatonin on glial cell activation and inflammatory cytokine expression; A - E, the protein expressions of ionized calcium-binding adapter molecule 1 (IBA-1), interleukin (IL)-1β, IL-6, and inducible nitric oxide synthase (iNOS) detected by western blotting; data showing mean ± standard error of mean; * P < 0.05, ** P < 0.01, *** P < 0.001
5. Discussion
The POCD is defined as changes in a patient’s mental activity, social activity, personality, and cognitive abilities after they were anesthetized or underwent surgery (26). The POCD is regarded as a major postoperative neurological complication that occurs in older individuals (27). The presentations of POCD include impaired attention, memory, language comprehension, and decreased social skills (28).
Inhalation anesthesia is linked to a higher incidence of POCD than intravenous anesthesia (29). Sevoflurane has become a first-line inhalation anesthetic among inhalation anesthetics due to its advantages of quick induction, moderate irritation, quick recovery, and stability maintenance (30, 31). Therefore, sevoflurane-induced cognitive dysfunction has become the focus of POCD research. The mechanism via which sevoflurane triggers POCD is observed to be closely linked to neuroinflammation and apoptosis (32). Therefore, understanding the mechanisms of sevoflurane-induced POCD and comprehending suitable targets and mechanisms to reduce or eliminate the occurrence of cognitive dysfunction is challenging. In this study, it was shown that melatonin could significantly improve the sevoflurane-induced cognitive impairment noted in elderly mice and attenuate the increase in autophagic proteins in the hippocampus, inhibiting neuronal apoptosis and neuroinflammation.
Melatonin is an indole neuroendocrine hormone with a wide range of biological activities (16) widely distributed in the human body. Melatonin’s secretion is regulated by the circadian rhythm in the suprachiasmatic nucleus of the hypothalamus. Melatonin can act on various systems in the body via multiple pathways and targets, has strong anti-inflammatory, anti-apoptotic, and anti-oxidative effects, and exerts neuroprotective effects. Studies have revealed that melatonin can improve chemical-induced brain damage by inhibiting oxidative stress, apoptosis, and autophagic mechanisms (33). The results of a large-sample, multicenter, controlled, randomized, and double-blind trial of elderly patients who underwent hip surgery (34) revealed that exogenous melatonin administration for 5 consecutive days significantly reduced the incidence rate of delirium 8 days after admission.
In this study, it was noted that the elderly mice experienced severe POCD after anesthesia with 2% sevoflurane, which caused a loss of cognitive function. The aforementioned results are consistent with the results of a previous study (35). However, aged mice showed improved cognitive function after treatment with melatonin, which was observed during the Morris water maze test. In comparison to the S-group mice, the S + M mice showed more platform crossings and achieved the target quadrant swimming time and distance. This finding indicates that melatonin can significantly reduce cognitive dysfunction induced by sevoflurane in aged mice. However, previous studies have also shown that the aging brain shows selective neurochemical changes involving several neuronal cell groups, and melatonin levels decrease during aging.
In the mouse model of Alzheimer’s disease, the elevation of brain melatonin significantly reduced the level of potentially toxic Aβ peptide. Exogenous compensation for melatonin loss during aging can promote central nervous system function by reducing metal-induced toxicity, lipid peroxidation, and cholinergic signal transduction loss. Therefore, whether exogenous melatonin supplementation can directly affect the cognitive function of aged mice through the above-mentioned mechanism still needs further exploration.
The activation of the anti-apoptotic effector Akt by PI3K can help promote cell survival (36). In most cases, the PI3K/Akt pathway transmits pro-survival signals in several cell types (37). Earlier studies highlighted that when rats were exposed to varying sevoflurane concentrations (e.g., 1.5% and 3%), it significantly elevated the level of pro-inflammatory factors (e.g., IL-8, IL-6, and tumor necrosis factor-α (TNF-α)) in the hippocampus of the rats. The experimental results also showed that the expression of the PI3K-Akt pathway-related factors was significantly inhibited, and dexmedetomidine treatment could suppress the inactivation of the pathway; however, this effect could be inhibited by YL294002 (i.e., the inhibitor of PI3K/Akt pathway) (38).
In this study, the findings showed that the 2% sevoflurane exposure inactivated the hippocampal PI3K/Akt/mTOR signaling pathway in mice, and this process was reversed by melatonin. The therapeutic effect of melatonin was partially reversed when combined with YL294002 and rapamycin (i.e., an mTOR inhibitor). The aforementioned results suggest that melatonin might improve POCD symptoms in mice via the PI3K/Akt/mTOR signaling pathway. In addition, earlier studies revealed that the hippocampal PI3K/Akt/mTOR signaling pathway negatively regulates autophagy-linked proteins; therefore, the downstream expression of autophagic proteins was further explored. The Western blotting results confirmed that melatonin could also inhibit 2% sevoflurane-induced autophagic activation in the mouse hippocampus by regulating the hippocampal PI3K/Akt/mTOR signaling pathway.
Neurons in the hippocampal region of the brain are critical cells for learning and memory (39). Neuronal apoptosis is considered a direct factor in POCD onset and advancement (40). Sevoflurane-induced neuronal apoptosis leads to cognitive impairment in rats (41). The caspase cascade is a vital component of apoptosis (42). A previous study has revealed that melatonin can alleviate secondary brain injury induced by cerebral hemorrhage in rats by inhibiting neuronal apoptosis (43). Therefore, the effect of melatonin on the mouse hippocampal neuronal apoptosis pathway was examined in this study. The findings of these experiments revealed that 2% sevoflurane significantly induced the up-regulation of Bax and cleaved caspase-3 (apoptotic proteins) and down-regulation of Bcl-2 (anti-apoptotic protein) in the hippocampus, accompanied by chromatin condensation and nuclear pyknosis, cell damage, and increased apoptosis. This phenomenon was significantly reversed by melatonin, signifying that melatonin exerted a protective effect against sevoflurane-induced nerve damage.
Neuroinflammation, particularly in the hippocampus, significantly contributes to POCD progression, mainly due to cytokines produced by the immune cells of the central nervous system (39, 44). It is widely accepted that activated microglia, which are important mediators of neuroinflammation, release pro-inflammatory cytokines in the hippocampus (45). High pro-inflammatory cytokine levels enhance excitotoxicity and lead to memory impairment. Previous studies have shown that sevoflurane anesthesia can significantly induce the up-regulation of IL-6, TNF-α, IL-1β, and iNOS expression in the hippocampus of aged rodents (46, 47). The inhibition of cytokines has been shown to effectively alleviate POCD (39). Therefore, the effect of melatonin on neuroinflammation was investigated in this study. The results indicated that melatonin could also inhibit sevoflurane-induced microglial activation in the hippocampus and the secretion of related inflammatory factors. A previous study has also reported that melatonin can ultimately reduce neuroinflammation by inhibiting lipopolysaccharide-stimulated microglial activation (48).
5.1. Conclusions
In conclusion, the open field and Morris water maze tests showed that melatonin significantly reduced neurological dysfunction in sevoflurane-anesthetized aged mice. The molecular experiment results suggest that this neuroprotective effect might be related to the restoration of sevoflurane-induced down-regulated PI3K/Akt/mTOR expression by melatonin treatment and the significant reduction of sevoflurane-induced apoptosis and neuroinflammation. This finding highlights the value of melatonin in the treatment of cognitive dysfunction and might provide a new strategy and basis for clinical drug treatment.