1. Background
Radiopharmaceuticals play a vital role in nuclear medicine, enabling physicians to diagnose and treat various medical conditions through imaging and targeting techniques that utilize radioisotopes. One important radiopharmaceutical is 99m technetium-pyrophosphate (99mTc-PYP), which holds significant value in nuclear medicine due to its bone-seeking properties. It has been widely used for the diagnostic imaging of bone diseases and certain cardiac conditions, such as amyloidosis (1-4). Hydroxyapatite and calcium phosphates in bone have a tendency to bind bisphosphonates, making these molecules preferred for bone scans. Most bisphosphonates have a hydroxyl group in a carbon position that binds to calcium phosphate with great affinity, and they also possess a highly reducing group with anti-adsorbing power in another carbon position (5-9). These properties make bisphosphonates preferred for diagnostic and therapeutic applications in nuclear medicine.
The first-generation bisphosphonates, such as etidronate, contain short side chains. The second-generation compounds, alendronate and olpadronate, contain aliphatic chains of varying lengths that include amino mass terminals. Third-generation bisphosphonates, such as risedronate and zoledronic acid, possess heterocyclic nitrogen side chains that are important for hydroxyapatite crystals in bone, and these agents are anti-resorptive. This anti-resorptive characteristic makes third-generation bisphosphonates at least 100 to 1000 times more potent than etidronate and pamidronate. At the cellular level, risedronate decreases bone turnover and inhibits osteoclasts (10-12).
Pyrophosphate (PYP), a molecule with an affinity for calcium ions found in bones, acts as a "bone seeker" when combined with radionuclides. Pyrophosphate is a phosphorus oxyanion composed of two phosphate units linked by a P-O-P bridge, with the molecular formula P2O74-. Pyrophosphate forms complexes with various metals, which is critical for its use in radiopharmaceuticals. It is highly soluble in water and exhibits a strong affinity for calcium ions, making it effective in targeting bone tissue where calcium deposition occurs (13-15). The absorption of four polar components by lanthanum-loaded biochar (BC-La), including phytic acid (IHP), adenosine-5′-disodium triphosphate (5-ATP), hydroxyethylidene diphosphonic acid (HEDP), and sodium PYP, was investigated by Yuan et al. (16). The results revealed that the maximum adsorption of BC-La for IHP, 5-ATP, HEDP, and PYP was 85.85, 9.04, 15.80, and 14.45 mg/g, respectively. Although the absorption of PYP is lower than that of bisphosphonates, the advantages of easier labeling with PYP and greater stability of radiopharmaceuticals with PYP-labeled agents make PYP an alternative to other bone-seeker ligands. An investigation (17) showed that labeling PYP with 177Lu achieved a maximum yield (> 99%) with only one minute of incubation at room temperature.
Emitters with low-energy β− are radionuclides such as 153Sm, 177Lu, 186Re, and 175Yb, which are applied for bone pain alleviation, while high-energy radionuclides such as 188Re, 90Y, and 166Ho are beneficial in marrow ablation due to bone composition (5-7, 10-12, 18-20). Another useful radionuclide is technetium-99m (99mTc). Technetium is a transition metal with the atomic number 43 and is primarily used in its isotope form, Tc-99m, in medical applications. Tc-99m, with a 6-hour half-life and gamma photon emission at 140 keV energy, is ideal for medical imaging. Technetium forms various chemical compounds and can exist in multiple oxidation states, with Tc (VII) and Tc (IV) being the most common in radiopharmaceutical chemistry. The chemistry of technetium is complex, allowing it to form stable complexes with ligands like PYP, enhancing its imaging capabilities (21).
Historically, 99mTc labeled PYP (Tc-PYP) has been widely utilized due to its excellent imaging properties, making it a cornerstone in diagnosing bone disorders and certain cardiac conditions, such as amyloidosis. This allows for accurate imaging and detection of abnormalities within heart tissue. The mechanism of localization involves an influx of calcium after cell death in acute myocardial infarction, leading to the formation of calcium phosphate complexes. These microcrystalline deposits act as sites for 99mTc-PYP uptake. When introduced into the body, PYP radiopharmaceutical accumulates in areas of increased bone metabolism or damaged bone tissue, making it ideal for identifying bone diseases and injuries such as fractures, tumors, infections, and cancer metastasis (15).
Rhenium is a transition metal with the atomic number 75, and its isotope, rhenium-188 (188Re), is of particular interest in therapeutic applications. Rhenium is in the same group as technetium in the periodic table, giving it chemical properties similar to technetium. Rhenium-188 has a half-life of 16.9 hours and emits high-energy beta particles (2.12 MeV maximum energy), effective for targeted radionuclide therapy. It also emits gamma photons (155 keV), applicable for imaging. Like technetium, 188Re exhibits multiple oxidation states, with Re (VII) and Re (V) being the most relevant for radiopharmaceuticals. The chemical properties of rhenium allow it to form stable complexes with ligands like bisphosphonates, ensuring effective delivery to target tissues while sparing surrounding healthy cells (22).
In this study, labeling PYP with 188Re is investigated. The combination of PYP labeled with 188Re enhances the capabilities of bone-seeker radiopharmaceuticals. Labeling PYP with 188Re involves attaching the radioisotope to the PYP molecule through specific chemical reactions. The labeling process is critical as it ensures stable binding between 188Re and PYP, allowing for effective radiation delivery to targeted sites within the bones. This study aims to explore methodologies for efficient labeling, evaluate the in vitro and in vivo stability of the 188Re- PYP complex, and assess its biodistribution and therapeutic efficacy in relevant animal models.
2. Objectives
By advancing the knowledge of 188Re-labeled compounds, this research contributes to developing novel therapeutic strategies for bone-related diseases, including metastatic bone cancer and osteosarcoma. Integrating diagnostic and therapeutic capabilities in a single agent presents a transformative approach in personalized medicine, offering targeted treatment while concurrently monitoring therapeutic outcomes. These dual capabilities could streamline patient management, enhance treatment efficacy, and improve predictive accuracy.
3. Methods
3.1. Materials
The necessary chemicals and PYP were obtained from Sigma-Aldrich. The compound underwent natural rhenium analysis to obtain UV spectra, which were recorded using a Varian Cary3 spectrometer. The Tungsten-188/188Re generator from PARS-Isotope Company of Iran was used as the source of 188Re. A saline solution (0.9% NaCl) was used to extract 188ReO4− from the alumina-based 188Re generator. A dose calibrator (Isomed, Germany) was used to measure the activity of 188ReO4−, which was 500 - 600 mCi. The radiochromatography analysis was conducted using Silica Gel ITLC chromatography paper from Agilent Technologies, US.
The activity distribution in the organs of mice was determined using the dose calibrator ISOMED 1010 (Dresden, Germany). The results were calculated as mean ± SD, and statistical analysis was performed using Student’s t-test, with P-values < 0.05 considered statistically significant. Male mice, with an average age of 8 ± 1 weeks, were sourced from the Nuclear Science and Research Institute (NSTRI) animal house. Animal studies adhered to the United Kingdom Biological Council’s guidelines.
All methods for preparation and quality control tests used in this investigation comply with the IAEA protocol for radiopharmaceutical production (23).
3.2. Production Along with Quality Control Assessment of the Rhenium-188-Pyrophosphate Compound
The experiment was conducted in two stages. The first stage involved the preparation of the compound in the cold phase using potassium perrhenate as a substitute for 188Re to obtain UV spectra. The second stage utilized 188Re to determine performance parameters, radiopharmacy processes, and biodistribution.
3.2.1. Cold Phase of Experiment
A solution of PYP with a concentration of 30 mg/mL was prepared by dissolving PYP in water. Different samples with varying amounts of PYP (5 - 22 mg), SnCl2.2H2O (0.2 - 6.0 mg), and ascorbic acid (0.3 - 7 mg) were prepared in vials. In the first stage, 0.08 mg of potassium perrhenate as a carrier in 1 mL of saline was added to each vial. The final solutions from the first stage were utilized to obtain the UV spectra of the compound for assessing its chemical properties.
3.2.2. Hot Phase of Experiment
In the second stage, 370 - 3700 MBq of 188ReO4− was added to the solution from the first stage. The pH of the solutions was varied between 3 and 8. The compound was shaken vigorously for 30 seconds. Product incubation was performed in a secured container for 30 minutes at room temperature. The solutions from the second stage were used to check radiochemical purity by the ITLC method and to assess biodistribution.
3.3. Radiochemical purity of Rhenium-188-Pyrophosphate
Samples with micropipette volumes (5 μL) were taken from the 188ReO4− solution and the radiolabeled complex, then dotted onto chromatography paper. Two solvent systems were utilized for the mobile phase in ITLC to discriminate the radiolabeled ligand from free 188ReO4− and 188Re2O4: (A) Saline solution (0.9% NaCl) and (B) acetone. The ratio of free 188ReO4− and 188Re2O4 to the radiolabeled 188Re-PYP was estimated.
In the saline solution, 188Re2O4 remained at the spot where it was applied, while the free 188ReO4− and the compound moved with the solvent front, allowing for the determination of the percentage of free 188Re2O4. In the acetone solution, the complex and 188Re2O4 remained at the spot, while the free 188ReO4− moved to the front, allowing for the determination of the percentage of free 188ReO4−. The percent radiochemical purity was obtained using Formula 1:
%Radiochemical purity = 100 - % 188Re2O4 - %188ReO4−
3.4. Investigation of In Vitro Stability of Rhenium-188-Pyrophosphate Compound
To study the compound's stability, it was incubated in human serum at 37°C and at room temperature for up to 72 hours and analyzed using ITLC. Rhenium-188-pyrophosphate (3.7 MBq in 100 μL) was added to 900 μL of freshly prepared human serum and stored at 37°C. At different time points (4, 24, and 48 hours after the reaction), 100 μL aliquots were removed and treated with 100 μL of ethanol (23). To precipitate the serum proteins, the samples were centrifuged at 3000 rpm for 10 minutes. Thereafter, chromatography of the supernatants was carried out.
3.5. Biodistribution of Rhenium-188-Pyrophosphate in BALB/c Mice
To assess the stability of the compound, it was incubated in human serum at 37°C and at room temperature for up to 72 hours and analyzed using ITLC. Rhenium-188-pyrophosphate (3.7 MBq in 100 μL) was added to 900 μL of freshly prepared human serum and stored at 37°C. At different time points (4, 24, and 48 hours after the reaction), 100 μL aliquots were removed and treated with 100 μL of ethanol (23). To precipitate the serum proteins, the samples were centrifuged at 3000 rpm for 10 minutes. Subsequently, chromatography of the supernatants was performed.
4. Results and Discussion
4.1. Preparation of Rhenium-188-Pyrophosphate
4.1.1. Cold Phase Results
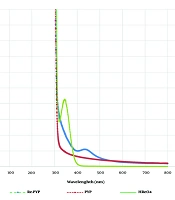
The UV-VIS spectrum is presented in Figure 1. Figure 1 shows the absorption spectrum of natural ReO4− in the presence and absence of PYP. In the spectrum of aqueous natural ReO4−, absorption was evident at 350 nm, showing a strong band, which is consistent with other investigations (24). However, in the presence of PYP, the λmax shifted to 450 nm. This phenomenon is related to the ligand-to-metal charge transfer (LMCT) from the ligand to the solvent in the complex, which stabilizes the bond between ReO4− and PYP. As 99mTc-PYP is widely used in clinical applications and the chemical properties of rhenium are similar to technetium due to their placement in the 7th group of the periodic table, it can be assumed that the binding between rhenium and PYP could be similar to the binding of technetium and PYP. Rhenium typically has oxidation states of +5, +3, or +1, although it also exists in an oxidation state of +7 in 188ReO4, produced in the reactor.
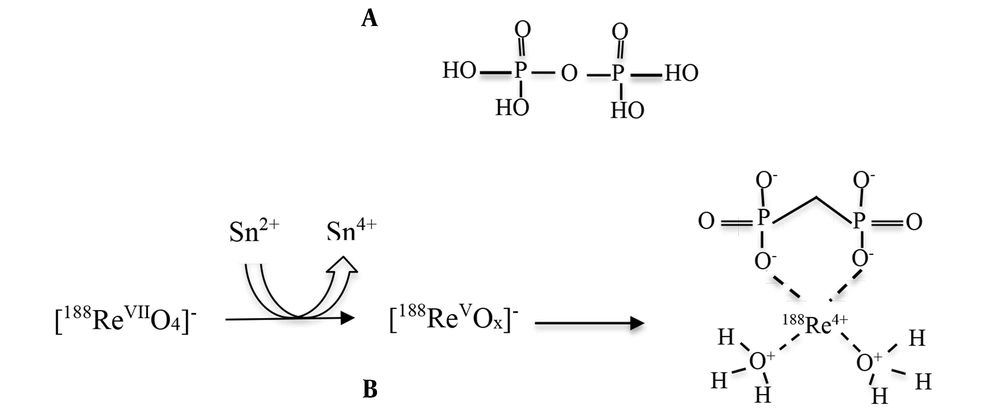
The good affinity of rhenium for nitrogen, oxygen, phosphorus, and sulfur in oxidizing conditions makes it possible to label ligands with rhenium (24). The procurement of 188Re radiopharmaceuticals is straightforward: a kit containing a reducing agent, often stannous chloride, and a weak chelating agent is treated with the generator eluate (ReO4−). The compound is then incubated for a short time, usually at room temperature, resulting in a pure therapeutic agent ready for injection. The possible formation and structure of 188Re-PYP are shown in Figure 2.
4.1.2. Hot Phase Results
Various amounts of PYP ligand, bulk SnCl2, and pH were tested to study the effect of these parameters on the reaction performance of the compound complexation and purity. In the first stage, natural rhenium, as HReO4, was applied. The amount of ligand varied between 4 and 20 mg for the 0.08 mg amount of rhenium as HReO4 solution. A 98.96% ± 0.1% complex yield was obtained using 10 mg of PYP, 1 mg of SnCl2.2H2O, 0.3 mg of ascorbic acid, and 0.08 mg of potassium perrhenate as a carrier in 1 mL with 370 MBq of 188ReO4− at pH 5 after 30 minutes of incubation at room temperature, leading to a specific activity of 30.22 mCi/mg.
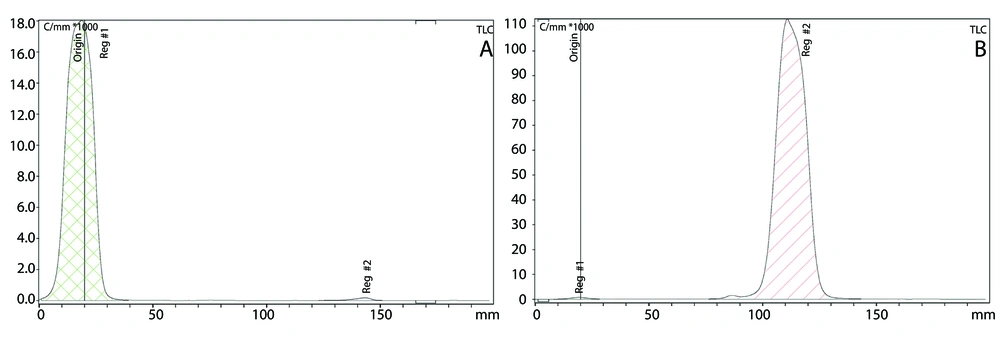
In the hot phase, a stability test was performed using two ITLC systems. For the first system, acetone was chosen as the mobile phase, where the Rf value for ReO4− ion was one, and for the compound with ReO2, it was zero (Figure 3A). For the second system, saline (0.9%) was the mobile phase, where the Rf value for ReO4− ion and the compound was one, and for ReO2, it was zero (Figure 3B). These two ITLC systems support the formation of the compound.
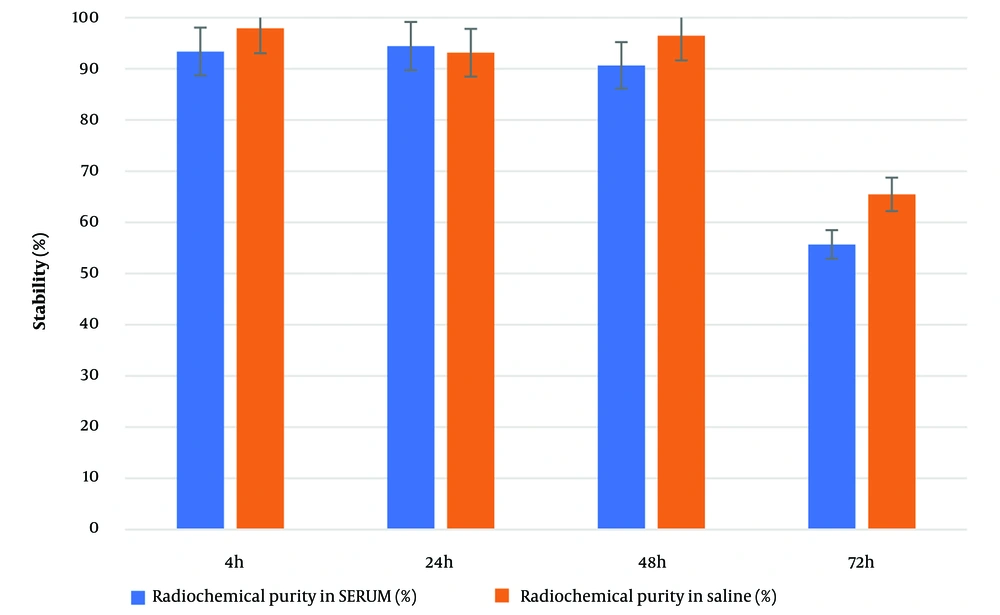
The radiochemical purity and complexation yield of the compound in human serum at 37°C and in saline over 72 hours are shown in Figure 4. The compound's stability in human serum was 90.86% up to 48 hours after complexation. This stability result up to 48 hours is comparable to the half-life of 188Re; therefore, it can be concluded that 188Re-PYP is stable during its period of efficacy.
4.2. Biodistribution Investigation
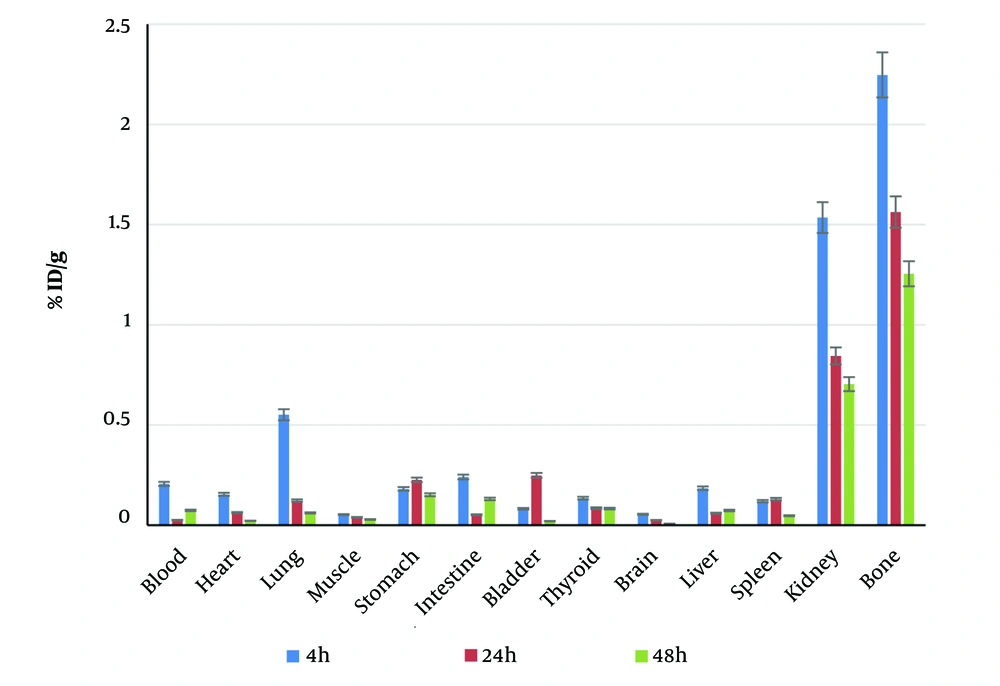
To evaluate the biodistribution of the radiolabeled ligand, BALB/c mice were utilized. The results, shown in Figure 5, confirmed that 188Re-PYP had maximum affinity for bone after 4 hours, with an uptake of 2.24% ± 0.667% ID/g. The maximum uptake for the kidney, spleen, and liver was 1.53% ± 0.378%, 0.13% ± 0.086%, and 0.18% ± 0.12% ID/g, respectively. The second highest uptake was in the kidneys, indicating that the radiopharmaceutical was eliminated through the renal system, while accumulation in vital organs such as the spleen and liver was negligible. These results reflect that the uptake of 188Re-PYP is primarily by bone and it is excreted by the renal system.
For comparison with other bone-seeker radiopharmaceuticals, the specific activities of 153Sm-EDTMP (7), 186Re-HEDP (6), and 90SrCl2 (16) were reported as 20, 35, and 40 mCi/g, respectively, while the specific activity of 188Re-PYP in this work was 30.22 mCi/g. This result shows that the specific activity of 188Re-PYP is comparable to clinical bone-seeker radiopharmaceuticals. The advantages of 188Re-PYP include easier labeling, with a maximum yield (> 99%) achieved by incubation for only a few minutes at room temperature, whereas other radiopharmaceuticals require at least 30 minutes of incubation at 100°C. Another advantage of 188Re-PYP is its in vitro stability, which is beneficial for clinical applications.
The difference in organ activity uptake between 188Re-PYP and 99mTc-PYP could be due to the application of a microfilter to remove any particles at the microscale, which reduced the activity of the solution by a factor of ten. The lower uptake in the liver might result from this filtration. Although the PYP kit used in this investigation is the clinical kit for 99mTc, in clinical evaluation, the 99mTc-PYP uptake of the liver is low enough to enable scanning of cardiac amyloidosis. Furthermore, an investigation by Jankovic et al. (25) showed that varying the molarity of PYP resulted in different liver uptake by a factor of 20, which was lower than the uptake in bone, similar to this investigation.
4.3. Conclusions
The present study investigated the initial labeling efficiency of 188Re-PYP, along with its biodistribution and in vitro stability. The 188Re-PYP conjugate was prepared under optimized conditions (10 mg of PYP, 1 mg of SnCl2.2H2O, 0.3 mg of ascorbic acid, and 0.08 mg of potassium perrhenate as a carrier in 1 mL with 370 MBq of 188ReO4− at pH 5 after 30 minutes of incubation at room temperature), revealing radiochemical purity and stability. The biodistribution of the compound in mice exhibited high affinity for bone, while the complex was eliminated through the kidneys.
To compare 188Re-PYP with 99mTc-PYP, it was noted that labeling with 188Re is more challenging than with 99mTc. In this research, it was necessary to increase the ligand-to-metal ratio to achieve maximum radiochemical purity. Labeling PYP with 188Re represents a significant advancement in the field of radiopharmaceuticals, particularly for therapeutic applications to alleviate severe pain arising from tumor metastases in bone. The incorporation of 188Re, a beta-emitting radioisotope, into PYP molecules extends its potential from purely diagnostic applications to include therapeutic uses. However, to fully establish the benefits of this radiopharmaceutical for clinical application, further experiments in larger animal models are needed.