1. Introduction
Primary small cell carcinoma of the ovary (SCCO) was first described in 1979 (1). It is a highly aggressive malignancy with a poor prognosis, which accounts for less than 1% of all ovarian cancers (2, 3). World Health Organization (WHO) classified SCCO as miscellaneous tumors (2014 edition). Two types of primary SCCO have been described, the hypercalcemic type (SCCOHT) and the pulmonary type (SCCOPT), which are clinically and histopathologically distinct entities. Both of these tumors are very uncommon in the ovary and SCCOPT is extremely rare with approximately 24 cases reported in the English literature to date. The majority of SCCOPT occurred in mature cystic teratomas, with only nine cases being described as “pure” primary SCCOPT (4). SCCOPT often occurs in elderly women (2). Clinically, it has many similarities with ovarian epithelial cell carcinoma (5), and preoperative diagnosis is difficult. Currently, most of the literatures described its pathological characteristics, clinical treatment and prognosis, and little attention has been paid to the imaging manifestations. With the purpose of reaching better understanding of the disease, we report a case of unilateral primary SCCOPT, the magnetic resonance imaging (MRI) manifestations and clinical data, and further reviewed relevant literatures.
2. Case Presentation
A 51-year-old woman was admitted due to lower abdominal pain and distension. No vaginal irregular bleeding was found. She had a history of breast cancer, and received radical mastectomy five years ago, followed by postoperative chemotherapy and targeted therapy. She had no genetic family history.
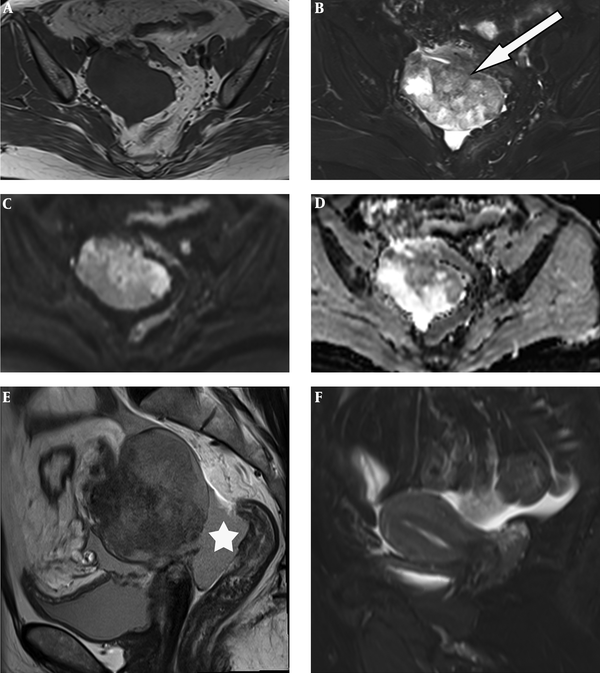
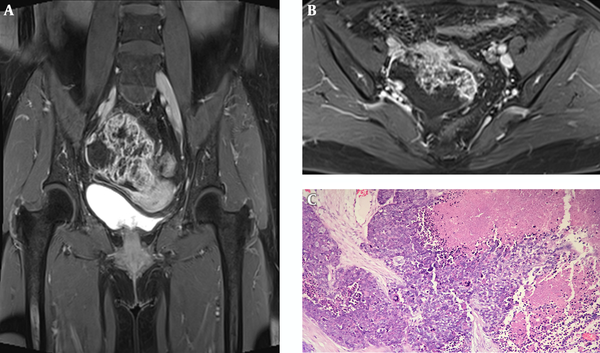
Table 1 summarizes the patient’s details including clinical data, imaging findings, pathological features and treatment. The serum cancer antigen 125 (CA125) level elevated to 68.79 U/ML (normal range 0 - 35.0 U/ML), cancer antigen 199 (CA 199) to 43.51 IU/ML (normal range 0 - 39.0 U/ML) and carcinoembryonic antigen (CEA) to 15.87 ng/mL (normal range 0 - 5.0 U/ML). Ultrasound of the pelvis demonstrated a complex mass extending to the right adnexa. Hence, MRI of the pelvis was performed, which revealed a 6.1 × 7.6 × 8.0 cm heterogeneous lobulated mass with solid and cystic components originating from the right adnexa. The lesion had iso- to hypo-intense signal on T1-weighted imaging (T1WI) (Figure 1A). T2-weighted imaging with fat suppression (T2WI FS) (Figure 1B) and T2-weighted imaging (T2WI) (Figure 1E) showed the solid component had slightly higher heterogeneous signal intensity (SI), higher than the SI of uterine myometrium, with hypointense foci; and the cystic component had the same SI as water. The solid component showed hyperintense on diffusion-weighted imaging (DWI) (Figure 1C) and hypointense on apparent diffusion coefficient (ADC) mapping (Figure 2D), with ADC values ranging from 0.77 × 10-3 s/mm2 to 0.95 × 10-3 s/mm2. The border between the lesion and the uterus, the sigmoid colon and adjacent small intestine was unclear (Figure 1B), while its posterior margin was well-demarcated with T2-hypointense capsule. Moderate pelvic effusion was observed (Figure 1E), and the endometrium was slightly thickened (Figure 1F). After administration of contrast media, the solid component of the lesion showed honeycomb-like intense enhancement (Figure 2A and B). The time-intensity curve (TIC) of dynamic enhanced was fast-rising platform type. No enlarged lymph nodes were detected. Computer tomography (CT) of the chest and abdomen showed no evidence of metastasis. We therefore suspected ovarian cancer and performed total abdominal hysterectomy with bilateral salpingo-oophorectomy. During operation, we detected a right ovarian tumor, which was adherent to the posterior wall of the uterus, as well as the right broad ligament, the sigmoid colon, and the small intestine. A nodule was detected in the left pelvic peritoneum. Intraoperative blood loss was 1300 mL.
| Details | |
|---|---|
| Sex | Female |
| Age, y | 51 |
| Symptom | Lower abdominal pain and distension |
| Laboratory examination | CA125: 68.79 U/mL; CA199: 43.51 IU/mL; CEA: 15.87 ng/mL |
| Imaging examinations | Ultrasound: a complex mass; MRI: heterogeneous lobulated mass with honeycomb-like intense enhancement |
| Immunohistochemistry | CD56 (+); synaptophysin (+); chromogranin-A (+) |
| Treatment | Surgical resection combined with postoperative chemotherapy |
Patients’ Characteristics
A 51-year-old woman with lower abdominal pain and distension. Unenhanced pelvic MR images. MRI of the pelvis shows a heterogeneous lobulated mass with solid and cystic components originating from the right adnexa. A, Axial T1 weighted imaging (T1WI) reveals the lesion had iso- to hypo-intense signal; B and E, T2 weighted imaging with fat suppression (T2W FS) and T2W imaging show the solid component has slightly higher heterogeneous signal intensity, with hypointense foci; the cystic component has the same signal intensity as water; C and D, Diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC) mapping exhibit restricted diffusion in the solid component; E, Moderate pelvic effusion is observed (*); F, Endometrium is slightly thickened.
Contrast-enhanced pelvic MR images and pathology. A and B, The solid component of the lesion shows honeycomb-like intense enhancement. Irregular regions of different sizes without enhancement are also seen; C, The malignant neoplastic tissue consists classic closely packed small cells with scant cytoplasm, hyperchromatic nuclei, and obvious cell atypia. Malignant component is separated by abundant fibrous tissue, and large necrosis area is present. (Hematoxylin & eosin staining, ×200).
Pathological examination revealed primary SCCOPT. Grossly, the right ovary measured 10.5 × 7.0 × 4.0 cm with lobulated margins. Microscopically (Figure 2C), a malignant solid neoplastic component was observed, which consisted of nests, trabeculae, and islands of closely packed small cells with scant cytoplasm, hyperchromatic nuclei, obvious cell atypia and mitotic figures. Tumor was separated by abundant fibrous tissue. Large necrosis area was also seen. Malignant tumors can be seen in the muscularis and adventitia of bilateral fallopian tubes, as well as in the peritoneal nodule. The uterus, intestine and lymph nodes (0/20) were not involved. Immunohistochemically, neoplastic cells expressed diffuse staining with neuroendocrine markers such as cluster of differentiation 56 (CD56) and synaptophysin. Chromogranin-A (CgA) and cytokeratin 20 (CK20) was focally positive. Ki67 index was approximately 50%. The patient was assigned an International Federation of Gynecology and Obstetrics (FIGO) ovarian cancer stage of IIB.
She underwent three cycles of taxol-carboplatin chemotherapy (TC) and myelosuppression occurred. The fourth pre-chemotherapy assessment revealed multiple metastases in the lungs and abdomen, and the chemotherapy regimen was changed to liposomal doxorubicin hydrochloride (40 mg) and carboplatin (0.5 g). Unfortunately, the tumor continued to worsen, and the following fifth PAC chemotherapy regimen (cisplatin 100 mg, liposome doxorubicin 40 mg, and cyclophosphamide 0.8 g) was also proved ineffective. Therefore, she received radiation therapy and genetic testing, which showed sensitivity to PARP (poly-ADP-ribose polymerase) inhibitors. Hence, she started to take oral olaparib tablets 300 mg twice a day, and anlotinib hydrochloride capsules 12 mg/day (two weeks for chemotherapy and one week for discontinuation, take medication periodically). For now, the patient has been followed up for 8 months after operation. During this period, she continued to lose weight, from 72 kg to 64 kg. Regular postoperative CT follow-up showed gradual increase in both number and size of liver and lungs metastases, accompanied by gradual increase of the serum CEA and CA199.
3. Discussion
SCCO is an extremely rare and aggressive ovarian malignancy, which accounts for less than 1% of all ovarian neoplasms (2). It may be growing rapidly, and sometimes, the clinical history may be short. The majority of patients are beyond stage I at presentation (6), and only around 20% to 25% have disease confined to the ovary. Two types of primary SCCO have been described, the hypercalcemic type (SCCOHT) and the pulmonary type (SCCOPT). SCCOPT was first reported in 1992 by Eichhorn et al. (2). It typically affects postmenopausal women with a median age of 49 - 59 years (4, 7). The clinical manifestations are usually atypical, including abdominal pain, bloating, and abdominal mass. A few may suffer from irregular vaginal bleeding.
Pathological morphology shows that most of the tumors are small round cells, with few cytoplasm, hyperchromatic nuclei and unobvious nucleoli, arranged in sheets, nests, islands and trabeculae. Neuroendocrine markers such as neuron specific enolase (NSE), CD56, CgA and synaptophysin (Syn) are usually expressed (6, 8). In our case, CD56 and Syn were positive, and CgA was partially positive.
To date, literature reports about SCCOPT are relatively rare, and most of them are related to its pathological characteristics, treatment and prognosis, with only few literatures emphasizing on its imaging features. Hence, radiologists have insufficient understanding about it. Due to its similarity with epithelial ovarian cancers in clinical symptoms and laboratory tests, it is not easily distinguished pre-operatively. Based on our patient’s pathological manifestations and previous literatures, we summarize the specific MRI findings of SCCOPT.
Approximately 25% - 50% of pure SCCOPT may involve bilateral ovaries (2). Most of the reported lesions are large in size and irregular in shape, with a maximum size of 30 cm. The majority of SCCOPT is ill-defined with lobulated or nodular appearance. Most literatures (7, 9-11) report it as predominantly solid with focal hemorrhage and necrosis (as some cases did not mention whether they existed or not, the incidence could not be calculated), while some other cases present as solid cystic masses (12), with the cystic part mostly being mucus, significant necrosis and cystic degeneration. No cases of cystic SCCOPT have been reported. But when combined with other tumors (such as serous or myxoid tumors or mature cystic teratoma), it could be mainly cystic (8, 13). Calcification has not been mentioned in the literature. Based on previous studies (9, 14) and our case, SCCOPT features iso- to hypo-intense signal on T1WI, with spot-like high-signal when hemorrhage is present. On T2WI, the lesion shows mixed signals, mainly slightly higher or intermediate signals. T2WI FS of the solid component shows slightly higher signal with focal low signal, and the cystic part shows T2 isointense to water. DWI and ADC mapping show restricted diffusion in the solid component (hyperintense on DWI and hypointense on ADC mapping) due to hypercellularity of cancer cells and high nuclear-cytoplasmic ratio. On enhanced scan, the solid component of the tumor enhances distinctly and persistently (14), which is different from the fast-in and fast-out type of enhancement of ovarian epithelial cancer. As malignant tissue is generally separated by fibrous stripes of varying thickness (2, 10), SCCOPT has a characteristic honeycomb-like enhancement. Pelvic effusion occurs in more than half of patients. Lymphadenopathy, as well as peritoneal, omental and mesenteric metastases are also common. When coexisting with other tumors, SCCOPT’s MRI manifestations are variable, which may lead to misdiagnosis. Moreover, it is difficult to distinguish with metastatic tumors when bilateral ovaries are involved.
Allowing for its rarity, SCCOPT remains a challenging tumor to treat. Standard primary surgical debulking is the treatment of choice, followed by adjuvant chemotherapy. There is no clear consensus for the optimal regime for postoperative chemotherapy because of the small number of patients and unavailability of follow-up data. Pelvic radiation remains enigmatic. Patients diagnosed with SCCOPT usually carry a very poor survival, generally dying rapidly within 2 years (6). There are also reports that indicate better prognosis when occurring with other tumors, which may be attributed to the small proportion of SCCOPT (15).
In our case, the imaging findings of the lesion are roughly consistent with the above literatures’ description. Unfortunately, because of our insufficient understanding of SCCOPT, we failed to make a precise diagnosis. The main differential diagnosis includes ovarian epithelial cell carcinoma and thecoma-fibromas. Ovarian epithelial cell carcinoma shares a similar age of onset, clinical symptoms, and laboratory tests with our patient, as well as certain MR manifestations. MR appearance of ovarian epithelial carcinoma is a variable combination of cystic and solid components, with the solid component exhibiting apparent enhancement and restricted diffusion. Different pathological subtypes have different characteristics (16): serous cancers and clear cell cancers are predominantly unilocular cysts with intermediate signal papillary projections inside; mucinous tumors feature different signal intensities of locules based on variable content; and endometrioid tumors are associated with endometrial hyperplasia or frank endometrial carcinoma in up to one - third of cases. The MR characteristics of thecoma-fibromas are hypo-intensity on both T1 and T2 weighted images. After administration of gadolinium, these tumors have variable enhancement depending on the proportion of fibrous cells and thecal cells, with reports of negligible and avid enhancement (17, 18). Moreover, hormones secreted by thecal cells may cause endometrial thickening, which was also seen in our patient.
In conclusion, when an ovarian tumor appears as a large, heterogeneous, complex and irregular mass, with the solid component exhibiting restricted diffusion on DWI and honeycomb-like persistent enhancement, SCCOPT should be considered. But as the number of case reports is small, and the imaging findings has a lot of overlap with other ovarian tumors, more data are needed to better understand its specific manifestations.