1. Background
Necrotizing enterocolitis (NEC) is the most common emergency in gastrointestinal pathology in neonates (1). NEC is still one of the major causes of mortality and morbidity in neonates and thus has become a priority for research (2, 3). However, the overall reported incidence of NEC among neonates is relatively low and ranges from 5% to 10% (3). NEC is characterized by inflammation and necrosis, starting from the mucosa and covering all layers. NEC is a progressive pathology of the gastrointestinal system (GIS) and is known as the disease of premature and low birth weight infants. The etiopathogenesis of NEC disease has not been fully identified. Although not certain, many factors such as prematurity, hypoxia, nutrition, pharmacological agents, infection, cytokines, and other factors causing deterioration of the intestinal barrier are responsible (4, 5).
Disease mortality is as important as morbidity. Intestinal adhesions, short gut syndrome and delays in the development of the nervous system can be seen after treatment of the disease. Acute spontaneous intestinal perforation is the main cause of mortality. The time it takes for nutrition to return to a healthy form determines the clinical course of hypoperfusion occurring in GIS (2). The terminal ileum region and proximal segment of colon are the most affected areas, but the disease can involve all GIS (1, 3).
The most important symptoms in NEC are cramped or blunt right lower quadrant pain, fever, watery or bloody stool, abdominal distention, nausea, vomiting, septic appearance, especially right lower quadrant sensitivity, decreased bowel sounds and a palpable mass. In the presence of neonatal NEC, the above findings may be accompanied by systemic findings such as apnea, lethargy, bradycardia and hypothermia. In advanced stages of the disease, erythema of the abdominal wall due to intestinal perforation and peritonitis, hematochezia due to severe necrosis, cardiovascular collapse, bleeding diathesis (consumption coagulopathy) may be seen. Although ultrasound (US) has a particularly diagnostic role in the pathologies of parenchyma, Doppler US is a more significant entity in demonstrating the absence of flow in the blood vessel (3, 4).
Although the disease was first described by Charles Billard in 1823, there are still weakly understood aspects of its etiology and pathophysiology (6-8). Even though many models have been used to understand this inflammatory process, the results obtained in neonate rat models gave the best explanations (8, 9). In neonatal rat models, enteral nutrition formulas, intestinal immaturity, hypoxia-ischemia were identified as major factors in developing the NEC environment (6-9).
The most important tools are abdominal X-ray, sonography, and color Doppler USE for the diagnosis. Early diagnosis and initiation of treatment are very important. Appendicitis, invagination, Hirschsprung’s diseases, volvulus, malrotation, spontaneous intestinal perforation, stress ulcer, meconium ileus, cow’s milk allergy colitis, Crohn’s disease, colitis ulcerous, leukemic or lymphomatous infiltration of the intestinal wall, bacterial or infectious colitis caused by opportunistic infections such as cytomegalovirus (CMV), pseudomembranous or enterocolitis due to antibiotics should be included in the differential diagnosis.
In recent years, the elastography technique has been widely used in the differential diagnosis of cases with tissue ischemia. Ultrasound elastography (USE) is a method that gives objectively measured tissue stiffness in gray scale or color image (10-12). Particularly for this process, the real-time wave elastography method was used. Elastography is the gold standard for the evaluation of liver necrosis.
2. Objectives
In this study, the value of the diagnosis of NEC is emphasized using the real-time tissue elastography.
3. Materials and Methods
Our study was approved by the local ethics committee (2014/04), which started with providing 16 male Wistar albino rats of the similar weight and age from the Giresun University animal research laboratory. These rats were divided into two groups (G-I and G-II) including eight rats each. The G-I group was taken as a control group and was not exposed to any stress. The G-II group was kept in medium containing 100% CO2 for 5 minutes and 4°C for 10 minutes. All processes were applied twice in a day for 4 days. We observed the elastographic data at 24, 48, and 96 hours in newborn rats.
3.1. Surgical Procedure
All rats were sacrificed by the end of the 4th day under ether anesthesia. In order to see inflammation in tissues macroscopically, first, the skin was cleaned with a povidone-iodine solution. Then the abdomen was entered through a median incision and a 2 cm terminal ileum segment was taken as a sample at a distance of about 1 cm to the ileocecal valve.
3.2. Radiological Assessment
The combination of gray-scale US and elastography was performed by a 4 - 13 MHz with an average of 12 MHz bandwidth linear probe in Esaote Ultrasonography Systems, MyLab60 model, produced in Geneva, Italy; with ElaXto imaging application.
Radiological procedures were performed by the same radiologist. After radiological examination, the elastography values obtained after the twenty-fourth and forty-eight hours were assessed by the radiologist for intestinal tissue stiffness of all rats. The same measurements were taken simultaneously in the intestine with NEC, as well as the intestine in the control group. Moreover, additional measurements were performed at 96 hours to better observe changes in advanced time.
The US evaluation showed a color map with the gray scale. The colored map occurred by green (soft) and red (hard) to display strain feature. The region of interest box was selected from the terminal ileum and soft fat tissue after the compression and decompression cycles. The strain measures were calculated automatically. This technique analyses the research area and adjacent surrounding tissues on the elastographic map. The strain value depends on qualitative USE data. Two discrete zones (A and B) are evaluated for quantitative outcome. The NEC parenchyma in area A was chosen as main research zone and area B was chosen as reference in the fatty area outside of area A. The strain measure was evaluated as the division of B/A. The elevated ratio depends on the hardness of the research area.
3.3. Statistical Analysis
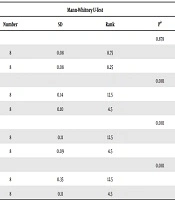
The study data were assessed with SPSS 20.0 package program (SPSS version 20.0, IBM, NY, USA). The frequencies and percentiles of the data are shown. Mann-Whitney U test was applied for variables to both groups that did not disperse normally during the examination of the differences. Wilcoxon Sign test was applied for the evaluation of differences between the measure periods of variables achieved from different periods. A P-value less than 0.05 was considered as significant statistically.
4. Results
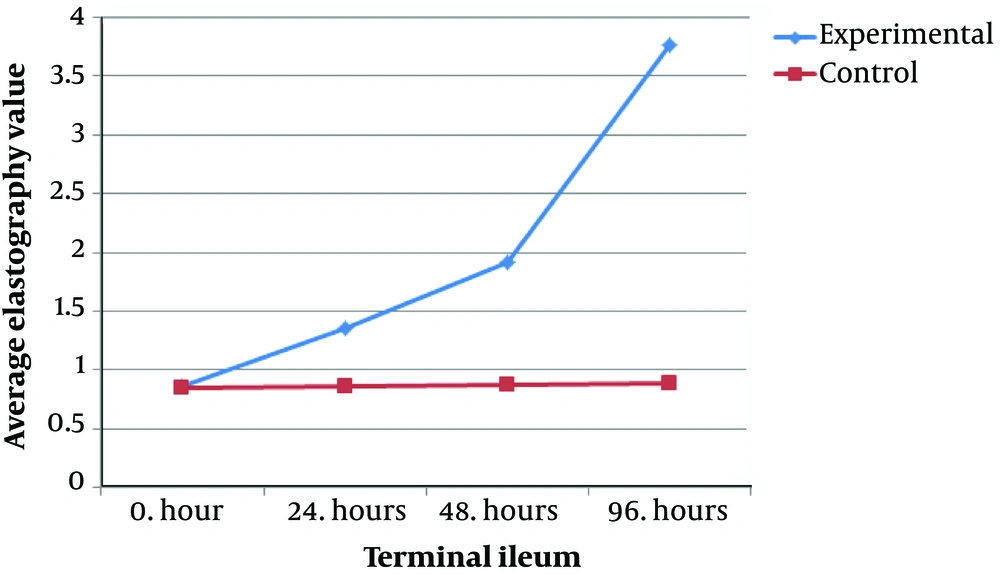
The elastography values at the terminal ileum at 24, 48, and 96 hours are given in Table 1. There were significant differences between the terminal ileum values of the study group at 24 and 48 hours, and 24 and 96 hours (P < 0.05) (Figure 1). As can be seen in Table 2, the terminal ileum values of the study group at 24, 48 and 96 hours were significantly greater than 0 hour (P < 0.05). Moreover, the terminal ileum values of the study group at the 96th hour were significantly higher than 48th hour values (P < 0.05). The reason for elastography values at the 96th hour were seen to be significantly greater than those at 0, 24, and 48 hours in the control group is dependent on stress induction. The reason for this is a stress (i.e., the mother rats do not feed the newborn rats for 4 days which causes inflammation).
| Mann-Whitney U-test | ||||
|---|---|---|---|---|
| Number | SD | Rank | Pa | |
| Terminal ileum (0 hour) | 0.878 | |||
| Experimental group | 8 | 0.08 | 8.75 | |
| Control group | 8 | 0.08 | 8.25 | |
| Terminal ileum (24 hour) | 0.001 | |||
| Experimental group | 8 | 0.14 | 12.5 | |
| Control group | 8 | 0.10 | 4.5 | |
| Terminal ileum (48 hour) | 0.001 | |||
| Experimental group | 8 | 0.11 | 12.5 | |
| Control group | 8 | 0.09 | 4.5 | |
| Terminal ileum (96 hour) | 0.001 | |||
| Experimental group | 8 | 0.35 | 12.5 | |
| Control group | 8 | 0.11 | 4.5 | |
Abbreviation: SD, standard deviation.
aP < 0.05.
Abbreviation: SD, standard deviation.
aSignificant.
Moreover, a complete relationship between the biopsy and elastography findings were detected. Elastography values of the terminal ileum of the control group at different ischemia times were assessed. There were no significant differences between the values in the 0th and 24th hours, and the 0th and 48th hours (P > 0.05). However, the values at the 96th hour were significantly greater than those at 0, 24 and 48 hours (P < 0.05) (Table 3).
| Friedman test | ||||
|---|---|---|---|---|
| Number | Mean ± SD | Test statistics | P | |
| Terminal ileum at 0 h | 8 | 0.085 ± 0.008 | ||
| 24 h | -0.968 | 0.0333 | ||
| 48 h | -2.324 | 0.0121 | ||
| 96 h | -3.679 | < 0.01 | ||
| Terminal ileum at 24 h | 8 | 0.086 ± 0.010 | ||
| 48 h | -1.356 | 0.0175 | ||
| 96 h | -2.711 | 0.004a | ||
| Terminal ileum at 48 h | 8 | 0.087 ± 0.009 | ||
| 96 h | -1.356 | 0.0175 | ||
Abbreviation: SD, standard deviation.
aSignificant.
5. Discussion
The disease often chooses the premature infant age group. Age groups in which gastrointestinal development regresses constitute the risk group for NEC (1-3). According to a study conducted worldwide, the incidence of NEC in infants called low birth weight (below 1500 g) was determined as 1% - 2% in Japanese, 7% in Austrians, 10% - 14% in Greeks, 14% in Argentina, and 28% in Hong Kong (3). This change in the incidence of NEC according to races reveals that there are multifactorial (environmental, genetic, biological) factors that determine the occurrence of the disease (3). NEC patients constitute 1% - 7% of the low birth weight infant emergencies who applied to the intensive care unit (ICU) in the USA (3).
The disease manifests itself with intolerance, abdominal tension and retention, and fever irregularities during feeding (1). It continues with findings such as vomiting, flank erythema, blood in stool, drowsiness, and apnea attacks. Patients are usually lost with disseminated intravascular coagulation (DIC) due to progressive systemic shock status as a result of oliguria and hypotension metabolic acidosis (1, 3, 6, 7).
For diagnosis, direct radiographic imaging of the abdomen is very important in suspected or intermittent cases. Detection of air between intestinal loops, blood in stool, and pneumatosis intestinalis are the gold standards for diagnosis (1). However, blood in stool is not specific for NEC. Among intestinal loops, gas appearance is present in 85% of patients (1, 4). Bowel loops occur when fermented bowel bacteria ferment the substrate bowel content. The ileocecal region and proximal colon are the most commonly affected sites, but the disease can involve all GIS. The most prominent pathological lesion in the intestine is coagulation necrosis or ischemic necrosis (1, 3, 4, 6, 7).
Disease formation is multi-factorial. The predisposition to viral bacterial diseases in prematurity, impaired tissue oxygenation in perinatal group patients, and the incidence of prematurity with congenital heart and lung diseases accelerate and increase disease formation. Although many clinical or experimental studies have been conducted on the pathophysiology of the disease, there are still many gaps that need to be filled in understanding. Including primary disorder of prematurity, enteral feeding and uncontrollable inflammation of the intestine have been accepted as tripartite (1, 4).
NEC has an average prevalence of 7% in infantile period with less than 1500 grams birth weight while NEC increases to 15% in infants weighing less than 750 grams (6, 7). The NEC protective aspect of breast milk appears when the disease is more frequently observed in enteral feeding neonates. NEC is basically an inflammatory disease. All stages of pathophysiological development of inflammation are valid for this disease. In the treatment, inflammation treatment was applied by combining the basic construct with surgery or without surgery according to the severity of the disease. Researchers have suggested some factors and mechanisms involved in the pathophysiology of disease formation, such as immaturity and consequent insufficiency or absence of intestinal mucin production (4, 8-12). In the visual examination of the operating segments, color change and tissue stiffness of intestinal loops, and edema can be macroscopically observed. Microscopically, in the early period, microvascular thrombosis, submucosal air cysts, vascular congestion, edema and inflammatory changes in the mucosal and submucosal area are observed. Microscopic pathology encountered in the late period is coagulation necrosis with or without hemorrhage and perforation (6, 7).
With the introduction of today’s technologies, the differential diagnosis of acute abdominal events has become much easier. Diagnostic techniques include abdominal X-ray, USG, B mode gray scale USG, nuclear medicine tools and the latest advanced tissue Doppler USG and USE. USE is a non-invasive imaging tool that can demonstrate the tissue hardness. The rule of USE depends on repetitive light compression by the US transducer device. Elastographic measurement methods include various techniques such as quasi-static Vibro-acoustography, transient elastography, acoustic radiation force impulse, shear wave elasticity imaging (SWEI), and supersonic elasticity imaging (SSI) in marketing (13-15). The elastography technique is able to differentiate normal and abnormal tissue in clinical applications. In addition, the technique is cheap, safe and easily repeatable.
Gao et al. (16) study in renal transplant allografts reveals that the real-time strain elastosonography is useful in assessing the progression of cortical fibrosis as a result of their study on 20 renal transplant patients. Also, Gao et al. (17) created an acute renal vein occlusion model in their experimental in vivo study. They emphasized that there was no significant difference in elastographic cortical thickness, however, when the ligation time of the renal vein is prolonged, there is a significant difference in the measurement of tissue strain and strain relaxation time.
Lin et al. (18) and Shi et al. (19) revealed that real-time elastography is an effective method for demonstrating fibrosis in the liver as a result of their study on 70 male Wistar rats and 62 New Zealand rabbits, respectively. Ramnarine et al. (20) indicated that elastography is able to quantify carotid plaque elasticity. The data obtained from our study revealed that the elastographic measurements of the tissue increase significantly when the ischemia time in the intestinal intestine is prolonged.
Dillman et al. (21) indicated that ex-vivo bowel wall shear wave elastography measurements increase when transmural intestinal fibrosis has existed as a result of their study on 12 patients with inflammatory bowel. In the study on rats conducted by Kim et al. (22), the distinction between the diseased colon and normal colon was found to be correlated with fibrosis level of elastography. Our study also supports these researchers because it was found that elastography values were significantly higher in the intestinal tissues undergoing ischemia (P < 0.05).
Also, in another experimental study on rats performed by Kim et al. (cited in Dillman) (23), it was detected that bowel wall elastography helps to distinguish acute inflammation from the fibrotic intestine in a Crohn’s disease animal model. Stidham et al. (24) reported that ultrasound elasticity imaging can differentiate the inflammation from the fibrotic intestine in rat models of inflammatory bowel diseases and can also differentiate between fibrotic and normal unaffected intestinal segment in a pilot study in humans with Crohn’s disease. Elastographic measurements obtained as a result of our study support the studies of these researchers. As the ischemia time extends to the 24th, 48th, and finally 96th hours, fibrosis in the intestinal tissue developing increases and there is a statistically significant difference in elastography values (P < 0.05). According to the results of our study, as ischemia time increases in the intestinal tissue, elastographic measurement values of the tissue increase significantly. This situation indicates that elastography can contribute to abdominal X-ray, sonography and Doppler USG used to assess the blood flow and ischemic tissues in the intestinal vessels lost or deteriorated in NEC.
Pediatric surgeons and clinicians distinguish NEC by its clinical status. The main causes of colitis are infective enteritis, Hirschsprung disease, ileal atresia, volvulus, meconium ileus, invagination, neonatal appendicitis, sepsis, and NEC. Clinical findings such as gastric retention with milk intolerance, apnea, lethargy, heat instability and hypotension constitute important conditions, thus the assisted diagnostic methods are very precious in differential diagnosis. In addition to these physical examination findings, we look at complete blood count, C-reactive protein (CRP), sedimentation, abdominal ultrasonography and color Doppler ultrasonography as auxiliary tests in diagnosis.
In this study, we created an experimental NEC model in newborn rats. We measured elastographic measurements at 24, 48, and 96 hours in live rats. Moreover, there was complete concordance between the biopsy and elastography findings.
Our aim in this experimental study was to pave the way for new research studies and consider elastography research in NEC diagnosis in clinical newborn babies. The result of the study which was performed on rats was promising for conducting further studies on the human neonates.
In conclusion, elastography is a reliable technique with superior features in the assessment of the terminal ileum in the neonatal NEC cases due to the width of its image window and better assessment of tissue stiffness with gray scale and color image. Therefore, it has been observed that the elastosonography imaging method can contribute to other diagnostic tests in the diagnosis of NEC. Our study has been done in rats and in small number; therefore, the generalization of the results to human neonates can not be done confidently at this point. We investigate the diagnostic performance of elastography, which is a new method for its use in this field in NEC that we experimentally created. The role of elastography requires a further studies in the future.