1. Background
Transjugular intrahepatic portosystemic shunt (TIPS) has become an effective therapeutic option to alleviate the complications of portal hypertension, including acute intractable variceal bleeding and refractory ascites (1-7). However, one of the main issues is the relatively high rate of stent dysfunction (25 - 50% of cases at 6 - 12 months after TIPS creation with bare stents), leading to recurrence of portal hypertension related complications (8-14). This high rate of shunt dysfunction made close monitoring of stent patency and frequent costly revisions mandatory, thus rendering TIPS creation a multistage procedure in most cases in which bare stents were used (15). Therefore, resolving stent dysfunction can reduce the rates of rebleeding and recurrent ascites after TIPS, and the frequency of required TIPS revisions. Among many theories, widely accepted ones are thrombosis, pseudointimal hyperplasia caused by bile leaks of transected bile ducts into the stent lumen, and intimal hyperplasia of the hepatic vein outflow tract (16-18). Recently, use of polytetrafluoroethylene (PTFE)-covered stents has decreased TIPS dysfunction by allowing endoluminal endothelial lining and preventing bile leak into the stent (19-27).
However, the predisposing factors leading to TIPS dysfunction, other than the use of bare stents, which are often the only available options, have not been elucidated well to date.
2. Objectives
In this study, to determine other risk factors for stent dysfunction, we investigated the clinical outcomes after TIPS creation with bare stents.
3. Patients and Methods
3.1. Patients
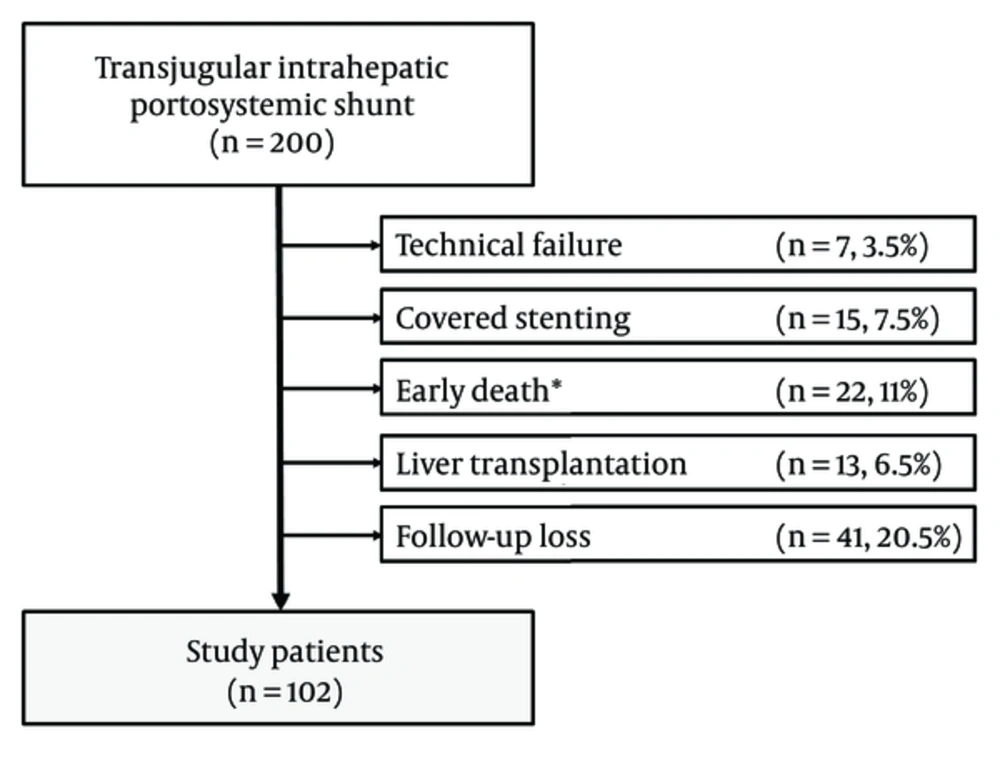
The institutional review board of our institution approved this retrospective study. The need for written informed consent was waived. Between January 1, 1999, and December 31, 2012, 200 patients who underwent TIPS were found from the medical records. We excluded 98 patients owing to technical failure to place TIPS (n = 7), having TIPS with covered stent (n = 15), early death < 1 month after TIPS (n = 22), subsequent liver transplantation (n = 13), and loss to follow-up (n = 41). We finally included 102 patients (80 men, 22 women; mean age, 60.1 years; range, 28 - 86 years) (Figure 1). The indications for TIPS and patient characteristics are shown in Table 1.
| Characteristic | Total (N = 102) |
|---|---|
| Age,y | 60.1 ± 11.8 |
| Sex, male/female | 80/22 |
| Underlying liver disease | |
| Viral (HBV/HCV) LC | 62 (55/7) |
| Alcohol LC | 30 |
| Cryptogenic LC | 6 |
| Othersb | 4 |
| Child–Pugh score | 7.7 ± 1.8 |
| Child–Pugh classification (A/B/C) | 31/53/18 |
| MELD score | 13.5 ± 4.1 |
| Indication | |
| Variceal bleeding | 83 |
| Refractory ascites | 19 |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; LC, liver cirrhosis; MELD, model for end-stage liver disease.
aValues are mean ± standard deviation or number of patients.
bNonalcoholic steatohepatitis and autoimmune disease.
3.2. Transjugular Intrahepatic Portosystemic Shunt
Before TIPS, computed tomography (CT) or ultrasonography was performed to plan the most suitable approach for the procedure. On the basis of our institutional guidelines, patients were required to have a maximum international normalization ratio (INR) of 1.5 and a minimum platelet count of 50,000/mm3. If preprocedural laboratory values did not meet these standards, they were corrected via proper transfusion. TIPS, however, was performed in patients with uncorrectable coagulopathy (INR ≥ 1.5 or platelet count ≤ 50,000/mm3) despite transfusion, especially on emergency cases such as variceal bleeding. TIPS was created through previously described standard technical methods (8, 10, 28).
TIPS was performed with conscious sedation and local anesthesia. Access to the right internal jugular vein was obtained with a micropuncture system under ultrasound guidance. The venotomy site was serially dilated, and a 9-F sheath was inserted into the right atrium. Then, preliminary right atrial pressure was measured. A 5 - F curved angiographic catheter was manipulated into a hepatic vein (usually the right or middle vein). After hepatic venography and pressure measurement, wedged hepatic venography was obtained to localize the portal vein, by using carbon dioxide (CO2) (29, 30) or an iodinated contrast agent. The intrahepatic portal vein was punctured from the hepatic vein with a 16-gauge Colapinto needle (Cook, Bloomington, Indiana, USA) under fluoroscopic guidance and then negotiated with a 0.035-in guidewire (Terumo, Tokyo, Japan). After catheter passage into the portal vein, main portal vein pressure was measured through the catheter and a direct portal venogram was obtained. Next, the parenchymal tract was dilated with an 8 - 10 mm (diameter) balloon catheter (Synergy or Mustang; Boston Scientific, Galway, Ireland) over a 0.035-in extrastiff Amplatz guidewire (Cook). The balloon was then inflated to reach an indicated pressure. Subsequently, a self-expandable uncovered stent (Wallstent [Boston Scientific, Natick, MA, USA] or Zilver [Cook]) was inserted. All stents except two 8-mm Wallstent and one 8-mm Zilver stents were 10 mm in diameter, with varying lengths. Furthermore, additional dilations with a balloon catheter 8-10 mm in diameter were performed whenever the pressure gradient was >12 mm Hg or reduction in the pressure gradient was < 25% (31). After measurement of final portal and right atrial pressures, portal venography was performed to assess patency and persistence of the varices. Varices were embolized after TIPS creation at the discretion of the primary operator, if there was persistent opacification of large varices after TIPS, or if the patient had clinical evidence of active variceal bleeding after stent placement.
3.3. Clinical Follow-Up
After TIPS, all 102 patients were followed clinically. Stent patency was assessed with Doppler ultrasound at 1, 7, and 30 days after TIPS creation; at 3-month intervals during the first year of follow-up; and every 6 months thereafter, or in cases of suspected shunt dysfunction. Recurrent variceal bleeding was defined according to the Baveno V criteria (32). Recurrent ascites was defined as the presence of clinically evident ascites or symptomatic hydrothorax (33). TIPS-associated hepatic encephalopathy was considered when de novo clinically evident hepatic encephalopathy developed or when previous encephalopathy worsened (34). The term “worsening encephalopathy” was applied to patients whose symptoms increased by at least one grade after TIPS creation, as previously reported (28).
3.4. Study Endpoints and Definitions
Stent dysfunction was considered when any one of the following events was shown: (i) recurrent variceal bleeding or ascites; (ii) abnormal Doppler ultrasound findings; or (iii) abnormal CT findings. Stent dysfunction on Doppler ultrasound was defined if the calculated peak velocity in the stent was < 90 or > 190 cm/s, and/or the change in peak velocity decreased by > 40 cm/s or increased by > 60 cm/s from baseline measurements. A decrease in portal vein velocity of > 30 cm/s from the baseline value was also considered as evidence of stent dysfunction (35-37). Stent dysfunction on CT was suspected if the stent showed filling defects or luminal narrowing. Stent stenosis due to neointimal hyperplasia or thrombosis was confirmed if there was a focal or diffuse filling defect that separated the column of contrast medium within the stent from the stent wall (38). Abnormal Doppler ultrasound or CT findings were confirmed with venography. Intervention and TIPS revision were performed if there was morphologic evidence of stent stenosis (i.e., ≧ 50% narrowing of the lumen) or occlusion on venography.
Stent patency was defined as the time interval between TIPS creation and stent dysfunction or patient death while having a patent stent. The stent was assumed to be patent at the time of death if there was no evidence of stent dysfunction. Patient survival was defined as the time interval between TIPS creation and patient death or last follow-up. If the patient was alive at the last follow-up, survival was considered equal to the follow-up duration. Primary patency was defined as a patent stent without need of reintervention.
Procedure-related major and minor complications were defined as previously reported (39), and treated accordingly. These complications did not include hepatic encephalopathy and stent dysfunction, which were recorded and analyzed separately.
3.5. Statistical Analysis
Data are presented as means ± standard deviations for continuous variables, and frequencies for categorical variables. Stent patency and patient survival were assessed by the Kaplan-Meier method and compared with the log-rank test results.
Risk factors for stent dysfunction were assessed by analyzing several demographic and technical variables. Demographic variables included patient age, sex, presence of hepatic encephalopathy, indication for TIPS (variceal bleeding or ascites), Child-Pugh class, and model for end-stage liver disease score. Technical variables included portal vein entry site (segmental branch or portal vein trunk access), pressure gradient after TIPS (≥ 12 or < 12 mm Hg), or stent type (Wallstent or Zilver). Segmental branch was defined as distal to the right and left portal trunks.
Significant variables for stent dysfunction in univariate log-rank analyses (P < 0.2) were entered into multiple analyses to determine significant independent factors. Multiple analyses were performed with a Cox regression mode. All statistical analyses were performed with SPSS software (version 21.0; SPSS, Chicago, Illinois, USA), and p-values less than 0.05 were considered statistically significant.
4. Results
4.1. Technical Outcomes
Of the 102 patients with technical success, 67 received Wallstent and 35 received Zilver. The portal vein access sites were the portal trunk in 56 patients and the segmental branch in 46 patients. The mean portocaval pressure gradient (23.5 mm Hg before TIPS creation) decreased significantly to 10.2 mm Hg immediately after TIPS creation (P < 0.001).
4.2. Complications
Six patients (6%) experienced procedure-related major complications including hemobilia (n = 2), hemolytic anemia (n = 1), sepsis (n = 1), subcapsular hematoma (n = 1), and pulmonary edema (n = 1). One patient with sepsis died of septic shock 44 days after TIPS creation. Hemobilia was discovered in two patients, both 3 days after TIPS creation. One patient was treated with endoscopic biliary drainage. In the other patient, hemobilia spontaneously resolved after conservative treatment. Intrahepatic biliary opacification was observed during TIPS creation in these cases. One patient developed hemolytic anemia with jaundice within 24 h of TIPS creation, which resolved within 2 months. Right hepatic subcapsular hematoma, which was caused by the wedged hepatic portogram with CO2 before TIPS creation, occurred in one patient and resolved with conservative treatment within 1 month. In one patient, pulmonary edema occurred within 24 h of TIPS, which resolved within 3 days.
4.3. Stent Patency
During the mean follow-up period of 1889 days, stent dysfunction occurred in 51 of 102 patients (50%). A total of 37 patients (36%) had undergone TIPS revision owing to clinical recurrence of portal hypertension-related complications such as variceal bleeding (n = 32) or ascites (n = 5). Among 14 patients who had not undergone TIPS revision, nine had recurrent esophageal or gastric variceal bleeding and were managed with endoscopic sclerotherapy. The other five patients (three with variceal bleeding, two with ascites) had no significant symptom recurrence despite stent dysfunction, and were conservatively observed.
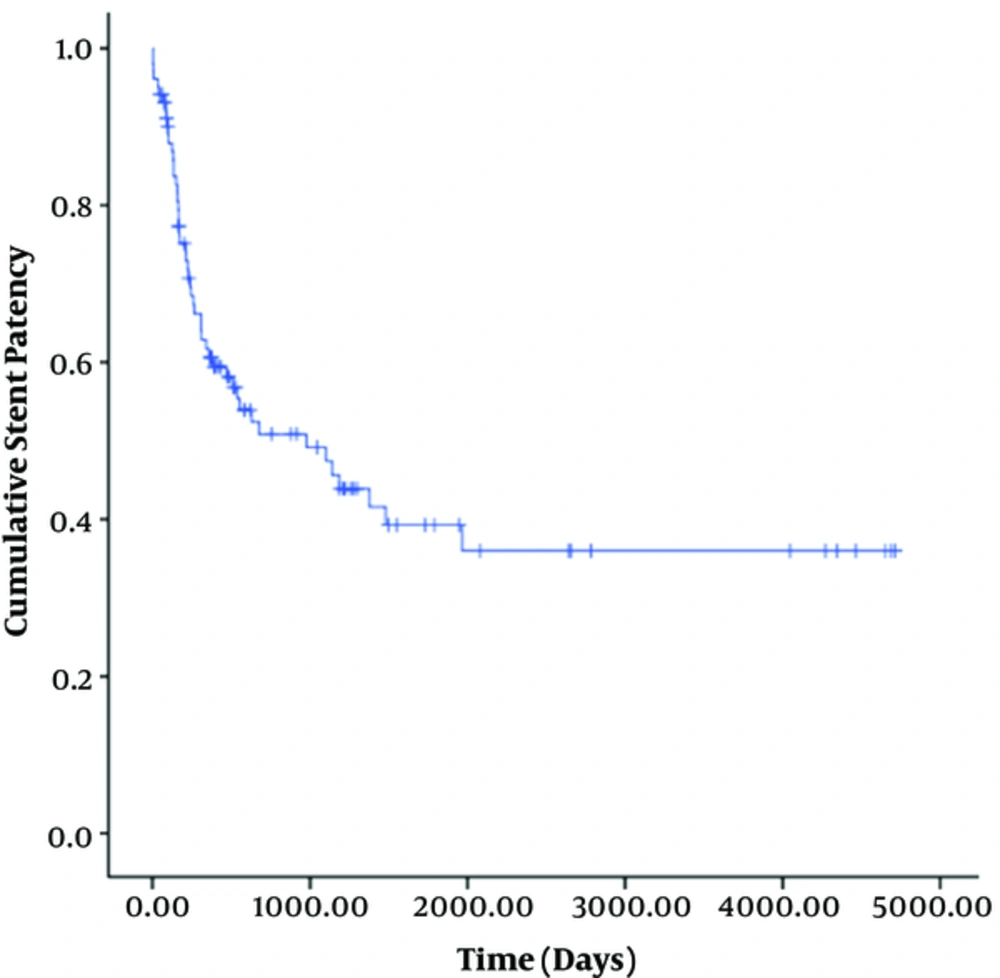
Stent dysfunction occurred due to stenosis (n = 32) and thrombosis (n = 5). The location of the stenosis in 32 patients was the parenchymal tract (n = 22), hepatic vein side (n = 8), or portal vein side (n = 2). The median stent patency time was 470 days (95% confidence interval [CI], 310-630 days) (Figure 2). The cumulative primary patency rates at 1 month, 6 months, 1 year, 3 years, 5 years, and 12 years were 96%, 71%, 55%, 36%, 19%, and 11%, respectively.
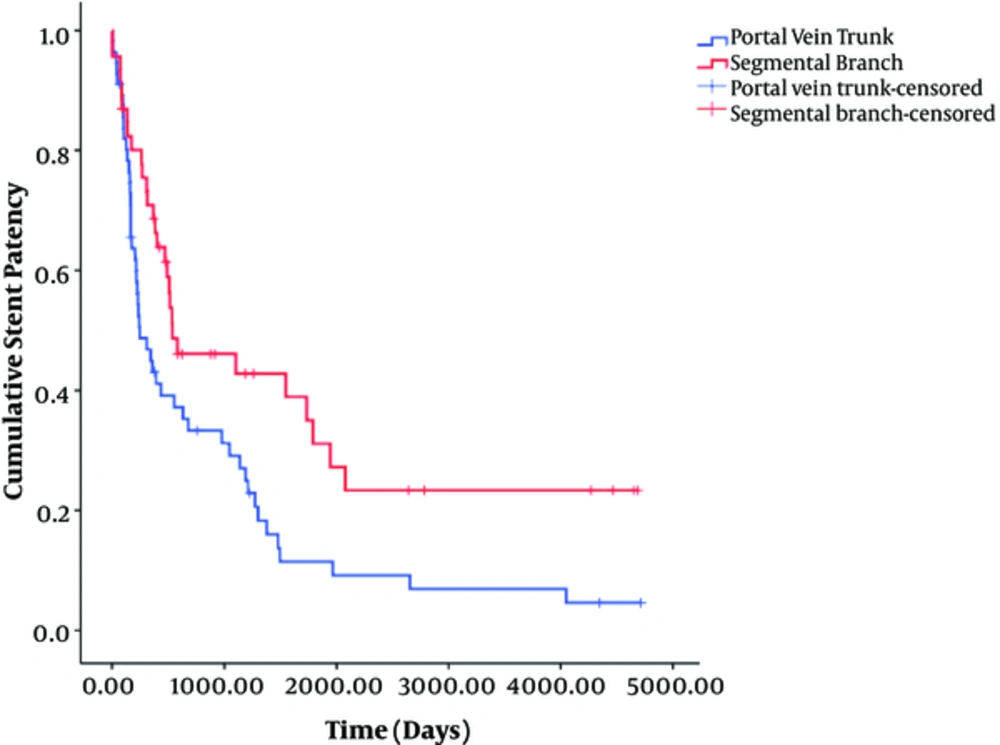
Univariate log-rank analysis showed that age ≥ 60 years (odds ratio [OR], 2.16; P = 0.142), Child-Pugh class B and C (OR, 4.901; P = 0.086), portal vein trunk access (OR, 7.215, P = 0.007), and Wallstent (OR, 3.323, P = 0.068) were significantly associated with shunt dysfunction (Table 2). Multivariate analysis showed that portal vein trunk access (OR, 2.561; 95% CI, 1.302 - 5.036; P = 0.006) was the only independent risk factor associated with stent dysfunction (Table 3) (Figure 3). Therefore, patients with portal vein trunk access had a 2.561 times greater risk of stent dysfunction than those with segmental branch access.
| Variable | Stent Dysfunction | Without Stent Dysfunction | P-Value |
|---|---|---|---|
| Mean age, y | 0.142 | ||
| < 60 | 25 | 31 | |
| ≥ 60 | 26 | 20 | |
| Sex | 0.771 | ||
| Male | 39 | 41 | |
| Female | 12 | 10 | |
| Hepatic encephalopathy | 0.412 | ||
| Yes | 41 | 35 | |
| No | 10 | 16 | |
| Indication | 0.808 | ||
| Variceal bleeding | 44 | 39 | |
| Refractory ascites | 7 | 12 | |
| Child-Pugh class | 0.086 | ||
| A | 12 | 19 | |
| B/C | 30/9 | 23/9 | |
| MELD score | 0.854 | ||
| < 13 | 28 | 23 | |
| ≥ 13 | 23 | 28 | |
| Pressure gradient | 0.747 | ||
| < 12 mm Hg | 33 | 33 | |
| ≥ 12 mm Hg | 18 | 18 | |
| Stent | 0.068 | ||
| Wallstent | 41 | 26 | |
| Zilver | 10 | 25 | |
| Portal vein entry site | 0.007 | ||
| Portal vein trunk | 35 | 21 | |
| Segmental branch | 16 | 30 |
MELD, model for end-stage liver disease
| Variable | Standard Error | Odds Ratio | 95% Confidence Interval | P-Value |
|---|---|---|---|---|
| Age ≥ 60 years | 0.346 | 0.660 | 0.335 - 1.300 | 0.230 |
| Child-Pugh class B and C | 0.454 | 0.479 | 0.197 - 1.167 | 0.105 |
| Portal vein trunk access | 0.345 | 2.561 | 1.302 - 5.036 | 0.006 |
| Wallstent | 0.405 | 1.102 | 0.498 - 2.438 | 0.811 |
4.4. Patients' Survival
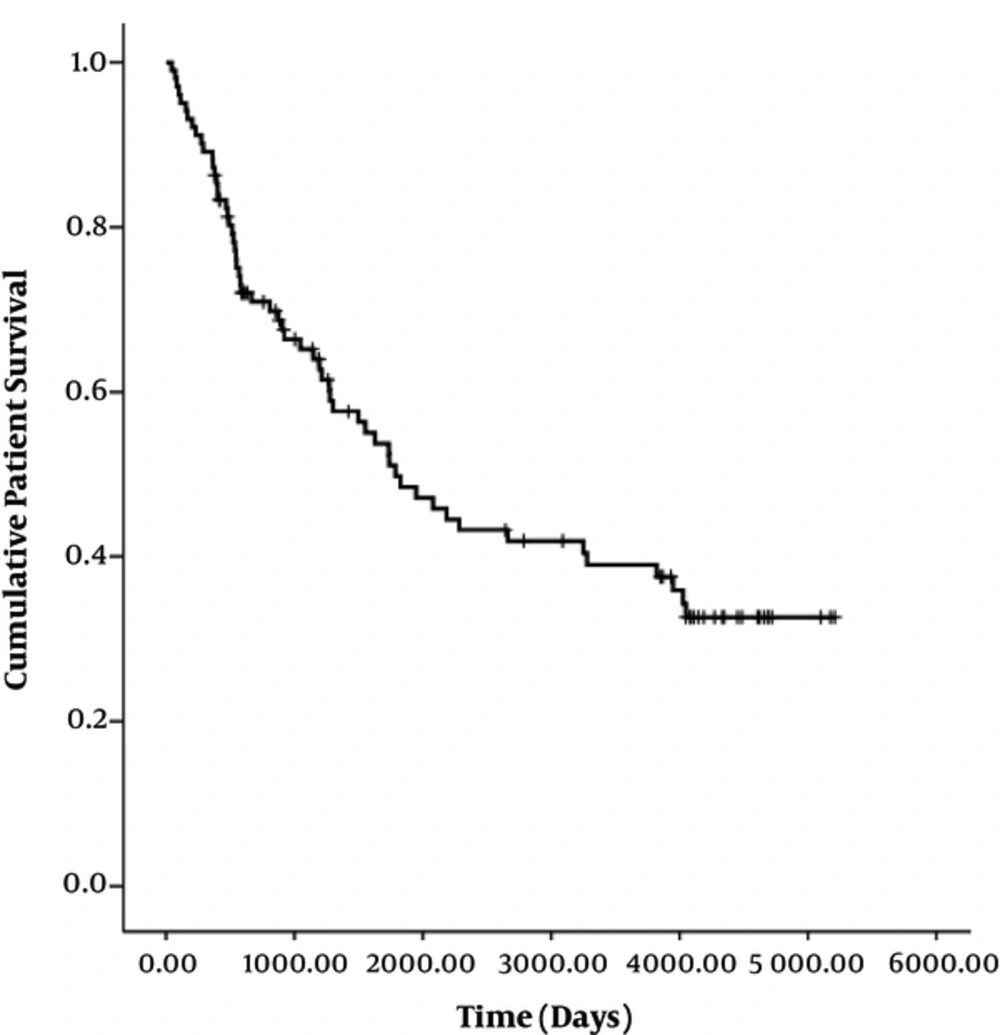
At the end of follow-up, 58 patients had died. There was no early mortality. The median survival time was 1783 days (95% CI, 1145 - 2421 days). The cumulative survival rates at 1 month, 6 months, 1 year, 3 years, 5 years, and 11 years were 100%, 93%, 87%, 65%, 49%, and 33%, respectively (Figure 4). There were no significant differences in median patient survival between segmental branch access (1947 days; 95% CI, 1416-2477 days) and portal trunk access (1734 days; 95% CI, 384 - 3084 days) (P = 0.648). Patient demographics and TIPS results according to the portal vein entry site are summarized in Table 4.
| Characteristic | Portal Trunk (n = 56) | Segmental Branch (n = 46) | P-Value |
|---|---|---|---|
| Mean age, y | 60.2 ± 11.2 | 59.9 ± 12.5 | 0.892 |
| Sex, male/female | 46/10 | 34/12 | 0.315 |
| Underlying liver disease | 0.808 | ||
| Viral (HBV/HCV) | 35 (31/4) | 27 (24/3) | |
| Alcohol | 17 | 13 | |
| Cryptogenic | 3 | 3 | |
| Othersb | 1 | 3 | |
| Child-Pugh score | 7.7 ± 1.8 | 7.7 ± 1.7 | 0.984 |
| Child-Pugh classification (A/B/C) | 19/27/10 | 12/26/8 | 0.654 |
| MELD score | 14 ± 4.4 | 12.8 ± 3.5 | 0.160 |
| Indication | 0.214 | ||
| Variceal bleeding | 48 | 35 | |
| Refractory ascites | 8 | 11 | |
| Type of stent | 0.002 | ||
| Wallstent | 44 | 23 | |
| Zilver | 12 | 23 | |
| Pressure gradient | |||
| Before TIPS | 22.9 ± 6.9 | 24.2 ± 8.0 | 0.370 |
| After TIPS | 9.7 ± 3.6 | 10.8 ± 5.5 | 0.236 |
| TIPS-associated HE | 16 | 10 | 0.431 |
| Follow-up timec | 2088.4 ± 1688.1 (44-5207) | 1643.9 ± 1581.5 (73-5173) | 0.176 |
| Outcome (survival/death) | 20/36 | 24/22 | 0.095 |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; HE, hepatic encephalopathy; MELD, model for end-stage liver disease.
aValues presented as mean ± standard deviation or number of patients.
bNonalcoholic steatohepatitis and autoimmune disease.
cData in parentheses are ranges.
4.5. Hepatic Encephalopathy
After TIPS placement, 26 (26%) patients exhibited at least one episode of hepatic encephalopathy: 24 patients developed a new episode of clinical encephalopathy and the remaining two patients experienced worsened encephalopathy with at least one grade increase. The mean time to onset of new or worsened symptoms was 12 days (range, 1 - 90 days). Out of these 26 patients, grade I encephalopathy occurred in 2 (7.7%), grade II in 20 (76.9%), grade III in 2 (7.7%), and grade IV in 2 (7.7%) patients. There were no significant differences in hepatic encephalopathy (P = 0.742) between segmental (10 of 46 patients, 22%) and portal trunk (16 of 56 patients, 29%) access sites.
5. Discussion
Although TIPS is an effective treatment for portal hypertension-related complications, a main problem lies in the unpredictability of long-term shunt patency. TIPS dysfunction is related to three common causes: acute thrombosis, pseudointimal hyperplasia in the intraparenchymal segment of the stent, and intimal hyperplasia of the hepatic vein outflow tract (16-18). The incidence of stent dysfunction in patients with TIPS created with bare metal stents is approximately 25% after 6 months or 50% after 1 year (8-14). To maintain TIPS patency, it is prudent to regularly monitor the shunt with Doppler ultrasound and eventually direct portography with frequent secondary intervention. However, this strategy is invasive and not cost-effective, and also does not completely protect the patient from further potential shunt dysfunctions with possible severe clinical portal hypertension manifestations. Accordingly, understanding the predisposing factors leading to TIPS dysfunction and efforts to solve those problems are necessary to maintain long-term patency after TIPS placement.
Currently, the best solution for TIPS dysfunction seems to be the use of polytetrafluoroethylene-covered stent grafts that isolate blood from hepatic tissue, therefore blocking the tentative triggering mechanisms of pseudointimal and intimal hyperplasia or thrombosis (19-27). Recently, a meta-analysis of six studies demonstrated not only a significant improvement of primary patency (hazard ratio [HR], 0.28) and a significant reduction of risk of hepatic encephalopathy (HR, 0.65), but also a significant decrease of mortality in the covered-stent group (HR, 0.76) (25). Moreover, in a recent randomized controlled trial by Perarnau et al. (27), 129 patients were divided to the covered-stent (n = 62) and bare-stent (n = 67) groups. The covered-stent group showed a significant 39% reduction in dysfunction compared with the bare-stent group (rate of dysfunction at 2 years: 44% for covered stent vs. 63.6% for bare stent).
However, there is little information on predisposing factors for TIPS dysfunction other than the use of bare metal stents. Thus, we sought to elucidate other potential reasons by assessing patients during a long period (14 years). For the first several years of performing TIPS at our institution, bare metal stents were exclusively used; however, after this period, both bare and covered stents were used. To maintain as much homogeneity in as many patients as possible, we only assessed patients receiving bare stents. Clark et al. reported that patients with the cephalic end of the stent extending to the hepatocaval junction showed longer stent patency than those with a stent terminating in the hepatic vein (40). Other investigators reported that failure of the caudal end of the stent to be parallel to the vascular wall of the portal vein probably induces portal vein stenosis (13, 41).
In this study, we carefully assessed TIPS stent dysfunction through hemodynamic measurements and strict long-term clinical follow-up. Our results are in accordance with other studies in terms of stent patency rate and location of stenosis (8-14, 27). Notably, we first revealed that portal trunk access (P = 0.006; OR 2.56; 95% CI, 1.3 - 5.04) was the only independent risk factor of TIPS dysfunction in multivariate analysis. The median stent patency with segmental branch access (538 days; 95% CI, 0 - 1204 days) was significantly longer than that with portal trunk access (245 days; 95% CI, 104 - 386 days) (P = 0.007). We assumed that this is owing to the relatively shorter length of the intrahepatic parenchymal segment of the TIPS stent in segmental branch access than in portal trunk access. In contrast, Cura et al. mentioned the possibility of kinking of the stent after peripheral punctures of the portal vein, leading to hemodynamically significant stenosis and an increased portosystemic gradient (13). However, in our study, there was no kinking of the stent in segmental portal branch access. Thus, our study presents important evidence for using the segmental portal branch when possible as a portal vein access site to maintain longer patency. Nevertheless, segmental portal branch access is not always possible, anatomically or technically, for all TIPS cases. Furthermore, there was no significant difference in patient survival, worsening of encephalopathy, or major complications between segmental access and portal trunk access.
The major limitation of this study is its retrospective design. Changes in technical aspects of the TIPS procedure occurred during this long-term study period. The second limitation of our study is that two different brands of bare stents were used (Wallstent vs. Zilver). Although a statistically significant difference between the portal trunk access and segmental branch access groups (P = 0.002) was noted and, in theory, the use of two different brands of bare stents could potentially introduce heterogeneity to the results, multivariate analysis did not show statistically significant differences in outcomes. Another limitation was the use of bare stents in light of the current guideline recommending the use of covered stents in TIPS creation (15). The reasons for analyzing patients with bare stents were that our analysis covered a 14 - year period and we sought to assess as many patients as possible. For the first several years of performing TIPS at our institution, bare metal stents were exclusively used; however, after this period, both bare and covered stents were used. To maintain as much homogeneity in as large a patient population as possible, we only assessed patients receiving bare stents. Finally, our investigation was conducted at a single institution. There are no generally accepted guidelines for TIPS surveillance, and also follow-up protocols differ across centers.
In conclusion, TIPS created with segmental portal venous access have superior patency over TIPS with portal trunk access. However, there was no significant difference in patient survival, worsening of encephalopathy, or major complications between segmental access and portal trunk access.