1. Background
The trauma of passing a large dialysis catheter through the vein wall is an important initiating stimulus for the fibrin sheath and subsequent fibrosis in the central vein (1). Respirations, cardiac motion, and positional changes tend to move the indwelling catheter within the vein, and can probably damage the wall as well (2, 3). Subsequent creation of an ipsilateral arteriovenous (AV) access with the associated high flows, turbulence, and perivascular vibrations stimulate intimal hyperplasia and these sequence of events ultimately result in stenosis of the central vein (4, 5).
Surgical management options have high primary patency rates, but they are prone to significant morbidity associated with exposure of the deep veins, especially considering the poor health status of most hemodialysis patients (6). Endovascular treatment options include percutaneous transluminal angioplasty (PTA) and percutaneous transluminal stenting (PTS).
Most studies on endovascular procedures for venous stenosis or occlusion in the hemodialysis patient have reported primary patency rates from 10 to 30% after 1 year (7-11). Published outcomes with stenting for central venous stenosis and occlusions have primarily utilized the Wallstent (Boston Scientific, Natick, MA, USA) with 12-month primary patency rates below 31% (8, 10-12), though one study demonstrated 56% patency (13). A study by Vogel and Parise (14) using the SMART stent (Cordis, Miami, FL, USA) demonstrated 67% primary patency for central venous stenosis at 12 months.
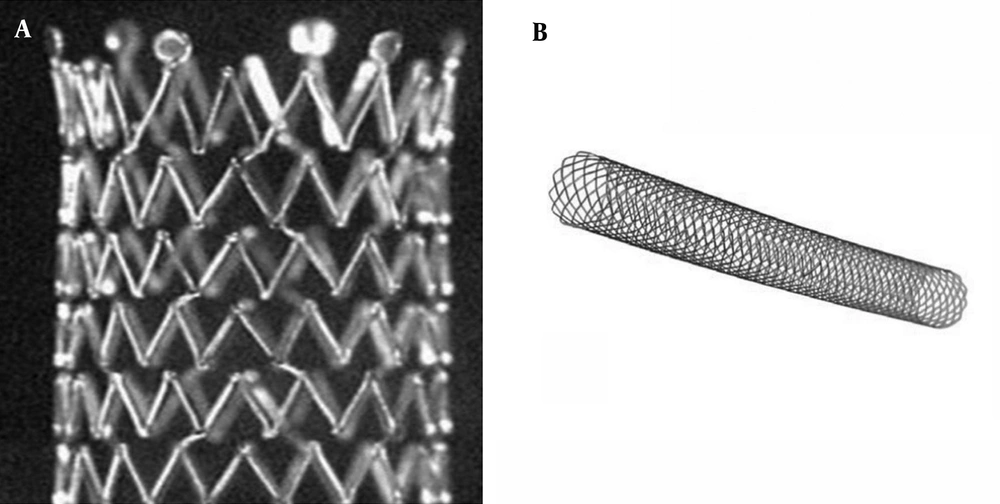
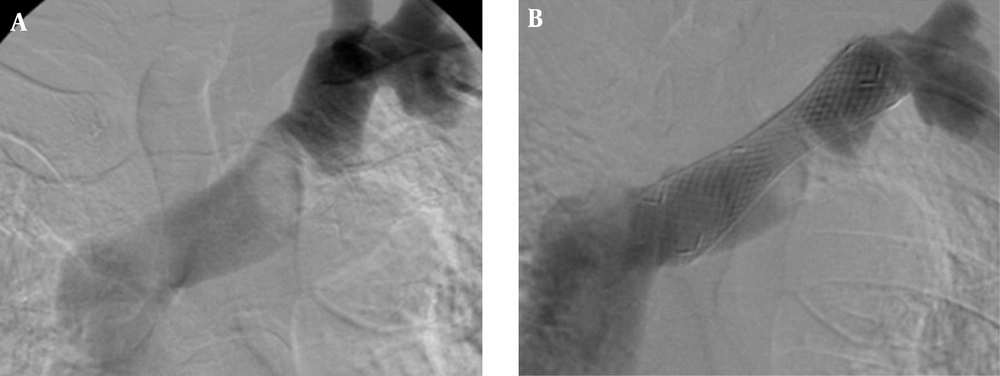
Depending on the density of the struts, stents can be classified as those with a closed-cell or an open-cell configuration (15). Closed-cell stents are characterized by small free cell areas between the struts, whereas open-cell stents have larger uncovered gaps. Flexibility and scaffolding are key characteristics derived from stent designs (15). Closed-cell stents are less flexible and may develop kinks and incomplete expansion (16). Conversely, stents with an open-cell configuration conform best to angulated vessels or tortuous anatomy.
Carotid artery stenting with different designs and configurations are available, but direct comparisons of open-cell versus closed-cell stents has rarely been performed in central vein stenosis or occlusions (16-18).
2. Objectives
The purpose of this study was to evaluate our outcomes following the use of an open-cell stent versus a closed-cell stent for patients with central vein stenosis or occlusions in hemodialysis patients and to compare long-term patency between the two groups retrospectively.
3. Patients and Methods
3.1. Patients Group
The records of 2418 hemodialysis patients who underwent endovascular treatment for central vein stenosis or occlusion at Gangneung Asan hospital and Soonchunhyang university hospital from 1997 to 2015 were reviewed. Symptoms included arm swelling and pain with venous hypertension. Patients with central vein stenosis or occlusions successfully treated with balloon angioplasty alone were excluded. A 70% or greater stenotic lesion of the central veins with filling of the collateral veins was an indication for balloon angioplasty. Stent placement was performed only if conventional balloon angioplasty was unsatisfactory, due to elastic recoil (> 30% residual stenosis and continued filling of collateral veins around the lesion) or occurrence of restenosis within 3 months after balloon angioplasty for the same lesion (19). When thrombus in the central vein was present, the treatment of choice was a primary stent placement.
Among them, 401 patients (200 males and 201 females; mean age, 59.17 years; range, 23 - 86) who underwent stent placement were enrolled in this study. Case data were retrospectively retrieved from radiographic reports, angiographic images, and medical records. Records were reviewed for the patient’s age, sex, incidence of diabetes, hypertension, smoking, and age of dialysis access. Radiographic records were reviewed for a history of previous angioplasty for the same lesion, location of venous stenosis or occlusion, and length and diameter of stents employed.
The causes of chronic renal failure were hypertension (32.4%), diabetes mellitus (32.2%), glomerulonephritis (7%), tuberculosis (0.2%), systemic lupus erythematosus (0.2%), pregnancy-induced intoxication (0.2%), and unknown origins (27.8%).
The clinical features of the patients whose central vein stenosis or occlusion was treated with an open-cell stent and those treated with a closed-cell stent are compared (Table 1).
| Characteristics | Open-Cell Stent (n = 257) | Closed-Cell Stent (n = 144) |
|---|---|---|
| Age (years ± SD) | 60.6 ± 12.3 | 56.7 ± 11.9 |
| Male/Female | 128/129 | 72/72 |
| History | ||
| Diabetes mellitus | 94 (37%)b | 35 (24%) |
| Hypertension | 190 (73%)b | 60 (42%) |
| Smoking | 10.5% | 10.9% |
| Duration of CRF (y) | 7.1 ± 5.7b | 6.5 ± 4.2 |
| Fistula | ||
| Rt/Lt | 74/183 | 45/99 |
| Age (m) | 48.4 ± 50.5b | 31.3 ± 31 |
| AVF/AVG | 141b/116 | 102/42 |
| Thrombosis (+) | 30 (11.7%) | 12 (8.3%) |
| PTA Hx | 108 (42%) | 57 (39.6%) |
| Central cath Hx | 146 (56.8%)b | 107 (74.3%) |
Abbreviations: AVF, arteriovenous fistula; AVG, arteriovenous graft; CRF, chronic renal failure; m, month; PTA, percutaneous transluminal angioplasty; Lt, left; Rt, right; y: year
aValues are presented as No. (%) or means ± SD.
bP value < 0.05
3.2. Procedure
All patients underwent an initial diagnostic fistulography by 21G needle into the outflow vein. The access circuit was evaluated via contrast digital subtraction fistulography from arterial anastomosis to the right atrium. Intravenous (IV) fentanyl citrate and midazolam were used for sedation in patients who were agitated or who could not tolerate the pain during the procedure. All patients with thrombosis were given IV 5,000 IU of heparin during the procedure.
Two hundred fifty seven open-cell stents and 144 closed-cell stents were used. Design and prototype of stents are summarized in Table 2. The stent used in each patient was based on product availability and physician preference.
| Stent Design and Prototype | No. |
|---|---|
| Closed-cell stents | 144 |
| Wallstent (Boston Scientific, Natick, MA), | 31 |
| Niti-S stent (Taewoong Med, Seoul, Korea) | 113 |
| Open-cell stents | 257 |
| Luminexx (Bard Peripheral, Murray Hill, NJ) | 27 |
| SMART stent (Cordis, Miami lakes, FL) | 125 |
| Zilver (Cook, Bloomington, IN) | 105 |
Stent sizes varied from 10 to 24 mm in diameter, and 20 to 80 mm in length. Open-cell stents with a diameter of 10 to 14 mm were placed in the study population. In closed-cell stents, the diameter varied from 10 to 24 mm. The stents were dilated post-deployment to the appropriate size of the vein in all cases. The diameter of the stent was determined after a review of baseline angiography results and the stent length required was based on the extent and location of the lesion so that both ends of the stent could be extended to the adjacent normal vein. Stents with a diameter 1 to 2 mm larger than that of the true vessel lumen were used for all devices.
3.3. Data Analysis and Statistical Analysis
The computerized access database was used to review all 2418 fistulography performed during the 14-year period from July 1997 to February 2011 retrospectively for central vein stenosis or occlusion in the hemodialysis patient.
Technical success was defined as a restoration of flow in the hemodynamic access with less than 30% residual diameter stenosis for the underlying central venous lesion treated and decreased collateral circulation (19). Primary patency was defined as the time interval from initial stent insertion to the next percutaneous intervention for central venous stenosis or occlusion (19). All complications that occured within 30 days were considered procedure related (19). Procedure time was defined as the time interval from the start of percutaneous puncture through the final post-treatment angiogram (19). Clinical evaluation and fistulography were performed if symptoms recurred.
A statistics software package (SPSS, version 14.0, SPSS) was used for statistical analysis. Measured values were reported as percentage or means. Kaplan-Meier method was used to calculate the cumulative probability of patency and mean patency. For analysis of categorical variables, the χ2 test and t-test were used (Table 1). The log-rank test was used to compare the patency between the two groups. A P value < 0.05 was considered statistically significant.
4. Results
4.1. Patients Group
When analyzing across both open-cell stents group and closed-cell stents group, we found that a history of diabetes, hypertension, duration of chronic renal failure, and history of previous catheter insertion were significant in the open-cell stents group (Table 1).
Considering the two groups collectively, the vessel most commonly treated was the innominate vein (n = 306), followed by the subclavian vein (n = 89), and jugular vein (n = 6). In the open cell-stent group, 205 innominate veins, 46 subclavian veins, and six jugular veins were involved, and in the closed-cell stent group, 101 innominate veins and 43 subclavian veins were involved. The author found 159 central vein stenoses, and 98 central vein occlusions in the open-cell stent group, and 78 central vein stenoses, and 66 central vein occlusions in the closed-cell stent group (Table 3). There were 30 cases of thrombosis in the open-cell stent group, and 12 cases in the closed-cell stent group.
| Location | Open-Cell Stent (n = 257) | Closed-Cell Stent (n = 144) | Total | ||
|---|---|---|---|---|---|
| Stenosis | Occlusion | Stenosis | Occlusion | ||
| Subclavian v | 28 | 18 | 16 | 27 | 89 (22.2) |
| Innominate v | 125 | 80 | 62 | 39 | 306 (76.3) |
| Jugular v | 6 | 0 | 0 | 0 | 6 (1.5) |
| Total | 159 | 98 | 78 | 66 | 401 |
Abbreviation: v, vein
aNumbers in parentheses are percentage.
4.2. Procedure
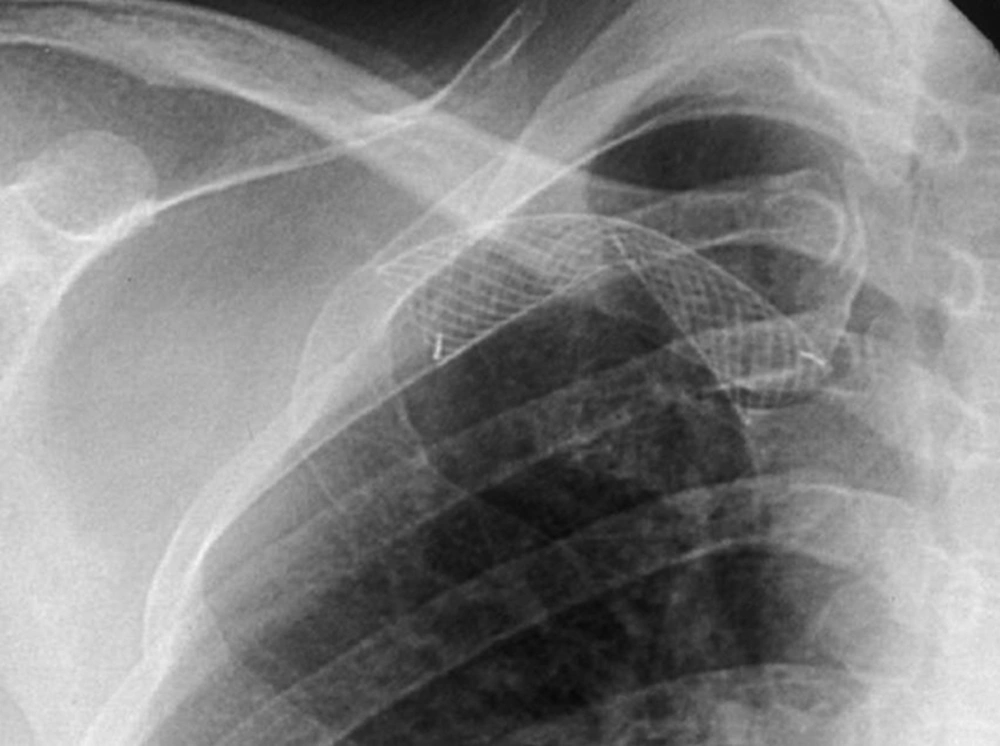
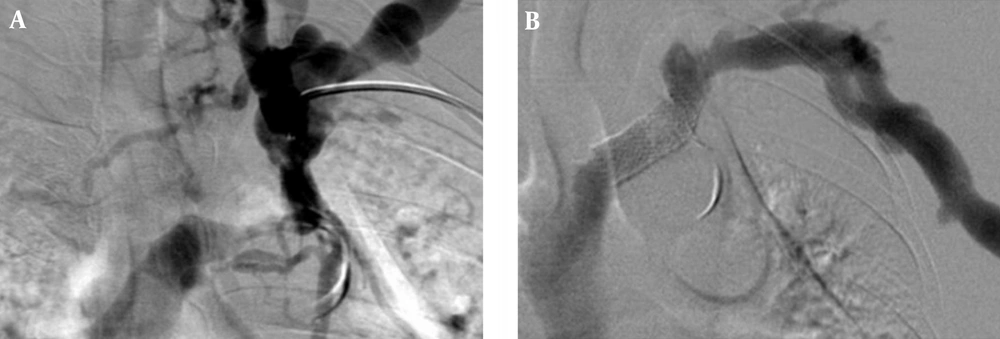
The technical success rate was 99.3% (398 of 401 procedures). One failure with an open-cell stent involved a successful stent placement, but coexistence of thrombosis in the graft site was not removed completely and the flow was insufficient. Another unsuccessful patient with a closed-cell stent was the result of incorrect stent positioning, thus creating a misplacement of the stent. One failure with the closed-cell stent was the result from stent collapse (Figure 1).
There were four cases of procedure-related complications. Two were complications from central migration of a stent during a placement that required an additional stent. A stent migration developed in two patients with closed-cell stents, one in the superior vena cava (SVC) and one in the pulmonary artery. In one patient with stent migration to SVC during procedure, the stent was successfully removed. The other stent had migrated into the right pulmonary artery after one day as post-PTS, but there was failure of retrieval. There were two cases of procedure-related vein rupture. In one patient with axillary vein rupture, balloon tamponade for 5 minutes was successfully performed. In another patient with innominate vein rupture, a stent was placed. There were no procedure-related or periprocedural deaths.
Mean procedure time was 53.2 minutes (range, 14-220). The insertion of one stent was sufficient to cover the entire lesion in each procedure. The mean follow-up period was 8.8 ± 9.97 month (range, 1-75). Follow-up fistulography was performed in 282 patients and only 21 patients had undergone clinical follow-up. There were three cases of technical failures, one case of death during follow-up, and 94 cases of no follow-up.
4.3. Data Analysis and Statistical Analysis
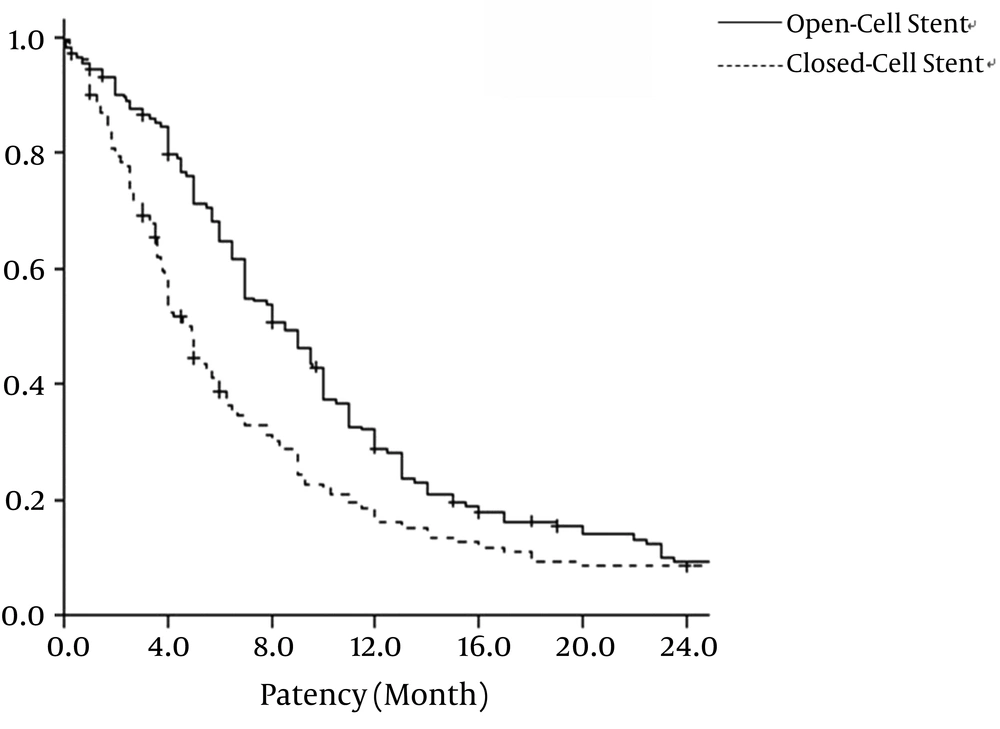
For the open-cell stent group, the primary patency rate was 86.4%, 64.8%, 28.8%, and 9.3% at 3, 6, 12, and 24 months, respectively. For the closed-cell stent group, the primary patency rate was 69.1%, 38.7%, 16.0%, and 6.5% at 3, 6, 12, and 24 months, respectively. Open-cell stents and closed-cell stents had mean patency rates of 10.9 ± 0.80 months and 8.5 ± 10.87 months, respectively (P = 0.002). The survival analysis of the primary patency rate for open-cell stent and closed-cell stent is show in Figure 2.
Primary patency rate of open-cell stent and closed-cell stent. Graph shows the patency rate of the central vein after placement of 257 open-cell stents and 144 closed-cell stents. Primary patency rate of open-cell stent group is significantly longer than that of closed-cell stent group (P = 0.02) by Kaplan-Meier analysis.
The mean patency rates in the open-cell stents group and closed-cell stents group are given in Table 4 by lesion site, lesion type (stenosis or occlusion), and presence of thrombosis. The mean patency rate for subclavian vein stenosis or occlusion was 11.1 ± 1.66 month in the open-cell stent group and 4.9 ± 0.18 months in the closed-cell stent group (P < .001). The mean patency rate for innominate vein stenosis or occlusion was 11.1 ± 0.92 months in the open-cell stent group and 10.1 ± 1.50 months in the closed-cell stent group (P = 0.15). The mean patency rate for central vein stenosis was 10.5 ± 0.87 months in the open-cell stent group and 8.0 ± 1.11 months in the closed-cell stent group (P = 0.042). The mean patency rate for central vein occlusion was 11.9 ± 1.59 months in the open-cell stent group and 8.9 ± 1.92 months in the closed-cell stent group (P = 0.020). In the case of thrombosis, the mean patency rate was 11.4 ± 2.02 months in the open-cell stent group and 10.6 ± 6.53 months in the closed-cell stent group (P = 0.153). In cases without thrombosis, the mean patency rate was 10.9 ± 0.85 months in the open-cell stent group and 8.2 ± 0.99 months in the closed-cell stent group (P = 0.006).
| Mean Patency (m) | P Value | ||
|---|---|---|---|
| Open-Cell Stent | Closed-Cell Stent | ||
| Lesion Site | |||
| Subclavian v | 11.1 ± 1.66 | 4.9 ± 0.18 | < 0.001b |
| Innominate v | 11.1 ± 0.92 | 10.1 ± 1.5 | 0.146 |
| Lesion Type | |||
| Occlusion | 11.9 ± 1.59 | 8.9 ± 1.92 | 0.020b |
| Stenosis | 10.5 ± 0.87 | 8.0 ± 1.11 | 0.042b |
| Thrombosis | |||
| (+) | 11.4 ± 2.02 | 10.6 ± 6.53 | 0.153 |
| (-) | 10.9 ± 0.85 | 8.2 ± 0.99 | 0.006b |
| Overall | 10.9 ± 0.798 | 8.5 ± 10.87 | 0.002b |
Abbreviations: m, month; v, vein
aValues expressed as means ± SD.
bP value < 0.05
5. Discussion
Central vein stenosis or occlusion is a common complication of the chronic hemodialysis patient, resulting in considerable edema of the arm with vascular access that is unable to drain normally. Surgical repair of these central veins can be difficult. That is why the percutaneous approach is preferable to the surgical approach (20). Angioplasty alone may represent a reasonable option for the treatment of central venous lesions. Stents are thought to add a beneficial effect to angioplasty by limiting the elastic recoil present in compliant veins, excluding damaged and dissected intravascular tissues, and acting as an intravascular support to counteract extrinsic compression (12). This is especially relevant for the central venous lesion, described as having high elastic recoil and poor results with PTA alone (12). It is natural to expect that reports concerning PTA as the only management option would have lower patency rates.
Previous findings in the patency rates of central vein obstruction in PTS vary widely from ours. We believe that the main reasons for this variation are the different PTS protocols, types of stents, study populations, age of access, access thrombosis at the time of intervention, and veins treated (8, 10, 12, 21, 22).
A stent can be classified as a closed-cell stent or an open-cell stent, depending on the density of struts (15). Closed-cell stents are characterized by small free cell areas between struts, whereas open-cell stents have larger uncovered gaps (Figure 3) (23). Closed-cell stents with a small cell size have a dense, metallic mesh and therefore, may provide more effective plaque coverage and reduce the risk of particle embolization (16). However, closed-cell stents are known to be less flexible and more rigid and are more likely to be used in straight morphologies (16). In contrast, open-cell stents are flexible; kinked lesions are ideally treated with these stents (16).
New stents were designed to incorporate visibility, flexibility, and expandability, permitting the treatment of the most complex venous lesion through endovascular means. Potential advantages of this approach include its less invasive nature, safety, ability to be done on an outpatient basis, better preservation of native veins, and, most important, its association with greater patient satisfaction and less discomfort (12).
Previous studies using closed-cell stents (Wallstent, Boston Scientific, Natick, MA) have failed to demonstrate improved survival or patency compared with angioplasty (7, 11, 12). Reports concerning the use of the Wallstent in central vein obstruction are common (13, 24, 25). With Wallstents, technical success was between 96% and 100%. Primary patency ranged from 42% to 84% at 6 months, but was less than 31% at 12 months in four studies (8, 10-12), with Haage et al. (13) demonstrating 56% primary patency at 12 months. Unfortunately, no one has since reported results as successful as those that the Haage group did.
Early experience with open-cell stents (Zilver nitinol stent, Cook, Bloomington, IN, USA) for central venous occlusion appears to indicate that they confer longer mid-term patency than historically observed results for central venous occlusion and central venous stenosis using Wallstents (22). In a study conducted by Vogel and Parise (14), use of the SMART stent demonstrated 67% primary patency for central venous stenosis at 12 months. In a study performed by Rajan and Saluja (22), use of the Zilver nitinol stent (Cook, Bloomington, IN, USA) demonstrated 66.7% primary patency for central venous stenosis at 6 and 12 months.
There have been two reports comparing the closed-cell stent with open-cell stent (26, 27). The authors of those reports reported no significant difference between the patency of the Wallstents and the nitinol-based Memotherm or Luminexx stent in their small groups (26, 27).
We have reported our large group study in stent treatment of central vein stenosis or occlusion with open-cell stent or closed-cell stent. Primary patency rates and clinical outcomes were comparable for the two-stent groups.
We found that the primary patency rate of open-cell stent was significantly higher than that of the closed-cell stent. The 6-month and 12-month primary patency rates with the open-cell stent were 64.8% and 28.8%, respectively, and primary patency rates with closed-cell stent were 38.7% and 16.0%, respectively. Open-cell stents and closed-cell stents had mean patency rates of 10.9 ± 0.80 months and 8.5 ± 10.87 months, respectively (P =0.002). The reported patency rate of 66.7% at 6 and 12 months following nitinol stent placement by Rajan and Saluja (22) might be the result of a small patient population (n = 6).
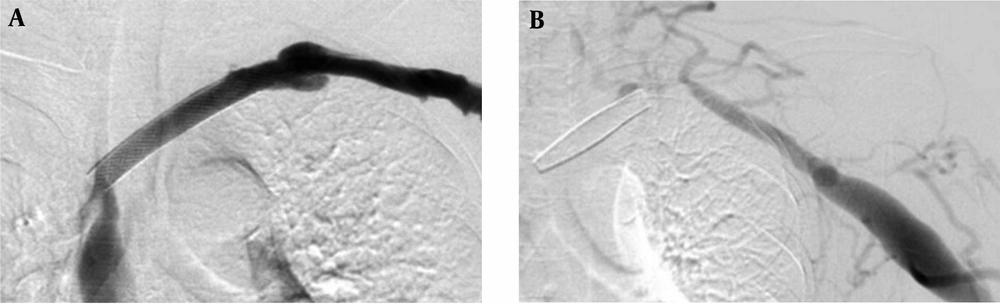
In this study, the mean patency rate of the subclavian vein with open-cell stent was 11.1 ± 1.66 months and the mean patency rate with closed-cell stent was 4.9 ± 0.18 months. There is a significant difference in the subclavian vein stenosis or occlusions between the open-cell stent group and closed-cell stent group in this study (P < 0.001). Our results might be related to characteristics of the open-cell stent, including flexibility, radial strength, and expansibility. The open-cell stent has physical properties that differ from those of the closed-cell stent. The closed-cell stent appears to have problems with its shortening and migrating during respiration (Figure 4) (8, 10, 11, 13).
The efficacy of stent deployment appears to vary by the vascular location of the stenotic lesion. Recently, nitinol stents have been introduced and have several physical characteristics that may confer longer patency compared with Wallstents (28). Nitinol, an alloy of nickel and titanium, exists in two temperature-dependent forms, which are predetermined by adjusting the ratio of nickel and titanium and through high-temperature heating. When nitinol assumes its higher-temperature form, it expands to its predetermined size and becomes more rigid. Nitinol is also superelastic in that it will deform its shape but return to its original configuration when an external force is applied and then removed (29-31). Shape memory and superelasticity allow for an improved apposition of the stent along the vessel wall and maintenance of radial strength (Figure 5).
The Wallstent is constructed of Elgiloy, the free ends are sharp and capable of embedding into the vessel wall. Eccentric loading of the Wallstent such as that produced by a stenosis results in concentric narrowing beyond the point at which the load is applied, making it susceptible to reduced wall contact and decreased radial strength (32). Poor or incomplete wall contact appears to be a risk factor for in-stent stenosis (33, 34), and decreased radial strength (29). In this study, there was a case of incomplete wall contact in the closed-cell stent group for stenosis at the left innominate vein (Figure 6). Closed-cell stent has also been observed to migrate and foreshorten in central venous segments (Figure 4) (8, 10, 11, 13). In addition, two cases of central stent migration were observed in patients with closed-cell stent in this study.
Open-cell stents are structured to allow for minimal foreshortening. Open-cell stents have become standard practice over closed-cell stents, mainly due to easier and more precise placement, non-shortening of the open-cell stents, and a longer vascular patency. The superelasticity and shape-memory characteristics of the nitinol stent improve flexibility, which also improve opposition to the vessel wall (Figure 5).
Depending on the density of the bridges between the different rings, nitinol stents can be classified into stents with a closed-cell or an open-cell configuration. Flexibility and scaffolding are key characteristics derived from stent design (15). Flexibility depends on the stent’s ability to conform the vessel tortuosity in the deployed state. Closed-cell stents are rigid, less flexible and may develop kinks and incomplete deployment in the tortuous vessel. Conversely, stents with an open-cell configuration conform best to angulated vessels or tortuous anatomy. Stents with a flexible and conformable open-cell configuration are preferred in vessels that are angulated or have a tortuous anatomy.
Patients in this study group did not experience any major morbidity or mortality, except for two cases of stent migration and minor complications.
There are several limitations to the present study. First, this study was retrospective and had mixed group patients among those with or without a history of hypertensio, diabetes mellitus, peripheral vascular disease, and use of tobacco, so their possible effects on patency rates could not be assessed. Although a history of diabetes, hypertension, duration of chronic renal failure and history of previous catheter insertion is more common in the open-cell stents group, the open-cell stent is more effective for treatment of central vein stenosis or occlusion in hemodialysis patients.
Second, differences in baseline and lesion characteristics may have influenced the observed results. It is unknown what role concurrent stenosis along the access circuit may have on patency following intervention for central venous occlusion.
In conclusion, the open-cell stent is effective for the treatment of central vein stenosis or occlusion in hemodialysis patients who have incomplete PTA results. These results suggest the use of open-cell stent for the treatment of central vein stenosis or occlusion in hemodialysis patients.